Abstract
BACKGROUND AND PURPOSE: West Nile virus (WNV) infection is an ongoing seasonal epidemic. We correlated the MR imaging findings with the clinical presentations and outcomes of WNV infection.
METHODS: We reviewed 14 brain and three spinal MR images: nonenhanced and contrast-enhanced T1-weighted images (T1WIs) and T2-weighted images (T2WIs), nonenhanced fluid-attenuated inversion recovery (FLAIR) images (11 patients) and enhanced FLAIR images (three patients), with diffusion-weighted (DW) images and apparent diffusion coefficient maps. WNV infection was diagnosed by means of enzyme-linked immunosorbent assay with a plaque reduction neutralization test. We also correlated the MR findings with the clinical presentation, course, and outcome to determine their prognostic importance.
RESULTS: MR imaging findings included: 1) normal (five patients); 2) DW imaging-only abnormalities in the white matter, corona radiata, and internal capsule (four patients); 3) hyperintensity on FLAIR images and T2WIs in the lobar gray and white matter, cerebellum, basal ganglia, thalamus and internal capsule, pons and midbrain (three patients); 4) meningeal involvement (two patients); and 5) spinal cord, cauda equina, and nerve root involvement (three patients). All patients with finding 1 and all but one with finding 2 recovered completely. Two patients with finding 3 died. Those with finding 4 or 5 had residual neurologic deficits that were severe or moderate to severe, respectively.
CONCLUSION: Patients with normal MR images or abnormalities on only DW images had the best prognosis, while those with abnormal signal intensity on T2WI and FLAIR images had the worst outcomes. No definite predilection for any specific area of the brain parenchyma was noted.
West Nile virus (WNV) infection is an ongoing seasonal epidemic in the US with considerable mortality and morbidity rates. The epidemic started in 1999, and the number of reported cases has substantially increased in the past 2 years. In 2003 alone, 9377 cases including 244 fatalities were reported (1). The geographic distribution of the disease expanded rapidly in 2002 and 2003 (2, 3).
Given these statistics, it is important to become familiar with the imaging features of WNV infection. The purpose of our study was to describe the MR imaging patterns of involvement in confirmed cases of WNV infection. We also correlated these findings with the clinical presentation, course, and outcome to determine the prognostic importance of each pattern.
Methods
From August 2002 to October 2003, we collected MR imaging studies and pertinent clinical findings in 17 patients from three institutions. Patients included in the study underwent neuroimaging as a part of the management of their clinical conditions, as referred by the clinical service. WNV infection was diagnosed by means of enzyme-linked immunosorbent assay. Confirmation of WNV identity was done with plaque reduction neutralization testing to detect specific immunoglobulin G antibodies (4–6).
Depending on their clinical presentation, patients underwent MR imaging of the brain (14 patients) or spine (three patients). MR images were interpreted at the time of the examination and reviewed again by a single neuroradiologist (Y.S.).
The examinations were performed with 1.5-T MR imaging machines. The brain MR imaging protocol included T1-weighted imaging (T1WI) before and after the intravenous administration of a gadolinium-based contrast agent, T2-weighted imaging (T2WI), fluid-attenuated inversion recovery (FLAIR) imaging before gadolinium enhancement in 11 patients and after enhanced in three, and diffusion-weighted (DW) imaging with apparent diffusion coefficient (ADC) maps.
Spine MR imaging protocols included: T1WI before and after intravenous gadolinium enhancement and T2WI.
The patients’ neurologic deficits at the time of discharge were graded as mild, moderate, or severe. Mild deficits did not limit the patients’ ability to perform baseline tasks. Moderate deficits limited their ability to perform some baseline tasks, but the patients remained independent. Patients with severe deficits could not perform at least some of their essential baseline activities so that they required help. Complete recovery was defined as a full return to their baseline functional level. We correlated these findings with the MR imaging patterns to determine the prognostic importance of the imaging results.
Results
Seventeen patients were included in the study. The patient population comprised six male and 11 female patients. Their average age was 51 years with a range of 18–80 years. Their overall mortality rate was 11.7%, and their average hospital stay was 14.7 days. The interval between the onset of symptoms and the MR imaging examinations was 2–13 days.
Five patterns were observed on the MR images. These included 1) normal brain MR imaging findings; 2) signal intensity abnormalities seen only on DW images, or isolated restricted diffusion; 3) increased signal intensity in the brain and brainstem on FLAIR imaging and T2WI; 4) meningeal involvement; and 5) intraspinal abnormalities consisting of spinal cord, cauda equina, and nerve root involvement.
Normal Brain MR Findings
Five patients with confirmed WNV infection had normal brain MR images. The interval between the onset of symptoms and MR examination ranged from 1 to 13 days. The patients’ symptoms included headache, altered mental status, dysphasia, fever, neck stiffness, malaise, arthralgias, and macular skin rash. All but one patient had a complete recovery. The only residual neurologic deficit was mild expressive dysphasia in one patient.
Isolated Restricted Diffusion
Four patients had MR imaging abnormalities only on DW imaging. One patient had bilateral diffusion restriction in the posterior limbs of internal capsule (PLIC) with bilateral extension to the corona radiata and the centrum semiovale. Three patients had bilateral diffusion restriction in the PLIC (right greater than left in two and symmetrical in the third), with additional involvement of the peripheral frontoparietal white matter. No corresponding abnormalities were seen on FLAIR images, T2WIs, or T1WIs. Images from two different patients illustrate these findings in the internal capsule (Fig 1) and in the corona radiata and subcortical white matter (Fig 2).
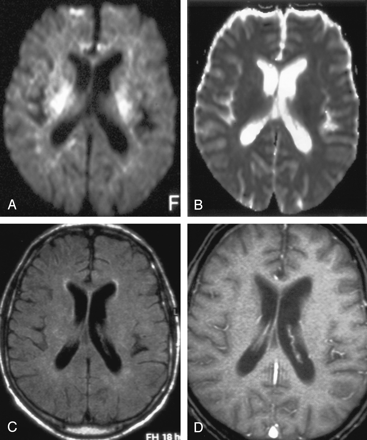
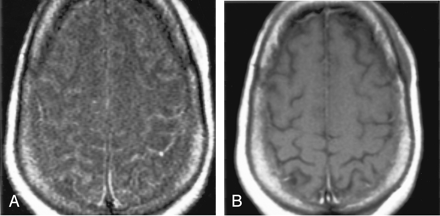
Patient with isolated restricted diffusion.
A and B, DW image (A) and ADC map (B) show restricted diffusion in the PLIC.
C and D, Findings on corresponding FLAIR image (C) and contrast-enhanced T1WI (D) are unremarkable.
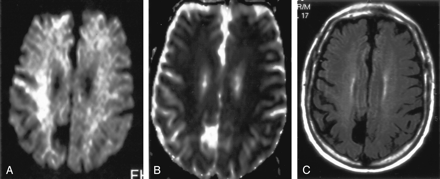
Isolated restricted diffusion in a patient who recovered without residual symptoms.
A, DW image shows asymmetric (right greater than left) high signal intensity in the superior corona radiata and subcortical white matter.
B, Corresponding ADC map shows subtle low signal intensity.
C, No abnormalities are present on the FLAIR image.
Presenting symptoms included headaches, lightheadedness, dizziness, agitation, confusion, nuchal rigidity, fever, chills, nausea, vomiting, arthralgias, and skin rash in various combinations. All patients in this group recovered without residual neurologic symptoms.
Brain and Brainstem Hyperintensity on FLAIR Imaging and T2WI
Three patients had abnormalities categorized as brain and brainstem hyperintensity on FLAIR imaging and T2WI. The first patient had abnormalities in the pons, midbrain, basal ganglia, thalamus, PLIC, meninges, and lobar gray matter and white matter. Changes included bilateral patchy areas of increased signal intensity on FLAIR images and T2WIs and corresponding decreased signal intensity on T1WIs. Some of these areas demonstrated restricted diffusion. No enhancement was noted in these areas on T1WI after the administration of a gadolinium-based contrast agent. Figure 3 illustrates several of these abnormalities with different imaging sequences.
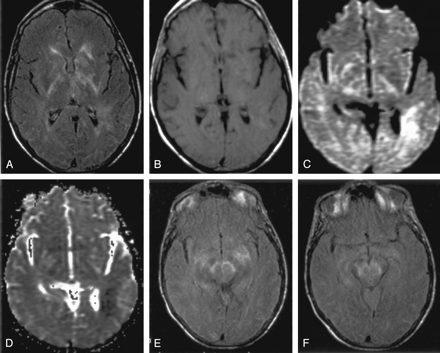
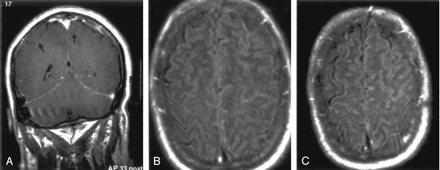
Images at the level of the basal ganglia and sylvian fissure in a patient with hyperintensity on FLAIR images.
A, Contrast-enhanced FLAIR image shows increased signal intensity in the basal ganglia and PLIC bilaterally, left thalamus, and left periventricular white matter, as well as the temporoparietal and occipital sulci.
B, Contrast-enhanced T1WI shows no corresponding enhancement.
C and D, DW image (C) and ADC map (D) show restricted diffusion is seen in the left periventricular white matter, right basal ganglia, and bilateral PLIC.
E and F, Contiguous FLAIR images show increased signal intensity in the midbrain and medial temporal lobes bilaterally, as well as in the right temporal lobe peripherally. Hyperintensity is again shown in the sulci, signifying WNV meningoencephalitis.
The second patient had bilateral patchy areas of increased signal intensity on the FLAIR images and T2WIs. These were observed in the pons, the midbrain, and the white matter of the posterior frontal and parietal lobes. The areas also had corresponding decreased signal intensity on T1WI. Patchy areas of restricted diffusion were also noted. No noticeable contrast enhancement was present on enhanced T1WI.
The third patient had more extensive abnormalities. FLAIR images and T2WIs depicted bilateral, asymmetrical, increased signal intensity in the gray matter and white matter of the frontal, parietal, and occipital lobes; the cerebellar hemispheres; and the midbrain. T1WI showed low signal intensity, with moderate degree of enhancement on contrast-enhanced T1WI. Some of these areas also demonstrated restricted diffusion. Figure 4 highlights the abnormalities in the cerebellar and occipital lobes in this patient.
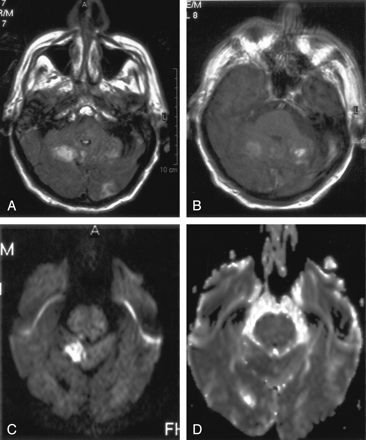
Another patient with hyperintense parenchymal abnormalities on FLAIR images.
A, FLAIR image demonstrates increased signal intensity in the bilateral cerebellum and in the left occipital lobe.
B, Contrast-enhanced T1WI shows the same areas of enhancement.
C and D, Corresponding DW image (C) and ADC map (D) show diffusion restriction in the right cerebellum.
Presenting symptoms included increasing somnolence in the first patient, agitation and loss of consciousness in the second patient, and sudden-onset high-grade fever and disorientation in the third. Two patients died during their hospitalization. The third patient, who had the most extensive involvement, had severe neurologic deficits after a prolonged (26-day) hospitalization.
Meningeal Involvement
One patient had diffusely increased signal intensity in the supratentorial sulci on gadolinium-enhanced FLAIR images (Fig 5A). No corresponding enhancement was observed in the sulci on gadolinium-enhanced T1WI (Fig 5B).
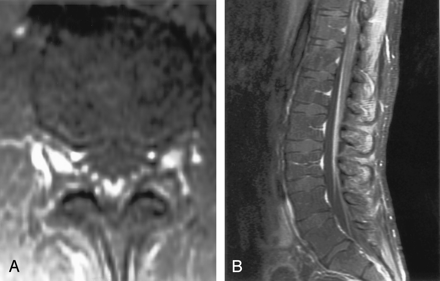
Patient with meningeal involvement.
A, Axial contrast-enhanced FLAIR image shows increased signal intensity in the sulci compatible with meningitis.
B, Abnormality is not appreciated on the corresponding contrast-enhanced T1WI.
The second patient had patchy, focal areas of increased enhancement in the parietal sulci and diffuse enhancement of the tentorial dura on gadolinium-enhanced T1WI (Fig 6A). Increased signal intensity was also seen on FLAIR images in the high frontal and parietal sulci (Fig 6B and C).
Patient 2 with meningeal involvement.
A, Coronal gadolinium-enhanced T1WI shows abnormal enhancement of the tentorial meninges and focal increased meningeal enhancement in the left parietal lobe. Arrows indicate subtle enhancement in the sulci.
B and C, FLAIR images show increased signal intensity in the sulci, more prominent on the left than on the right.
Presenting symptoms included increasing somnolence in patient 1 and high-grade fever, headache, and confusion in patient 2. Patient 1 had residual generalized and decreased muscle strength in the extremities, as well as slowing of speech; this patient was transferred to a rehabilitation facility after 19 days of hospitalization. Patient 2 had extensive memory deficits and was also transferred to a rehabilitation facility after a prolonged 21-day hospital stay.
Intraspinal Abnormalities
Three patients had intraspinal abnormalities on MR imaging of the thoracic and lumbosacral spine.
The first patient presented with fever and decreased strength (grade 1/5) with progressive weakness in both lower extremities. Contrast-enhanced T1WI imaging showed enhancement of the cauda equina and lumbosacral nerve roots (Fig 7). The patient was discharged to a rehabilitation facility after 10 days of hospitalization. He had residual weakness and foot drop in the right lower extremity.
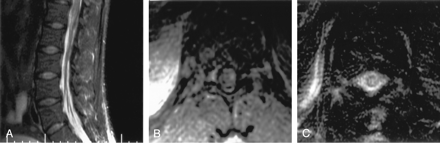
Patient with intraspinal abnormalities.
A, Axial contrast-enhanced T1WI through the region of the cauda equina shows marked abnormal enhancement of the nerve roots, which appear as bright dots in the thecal sac.
B, Sagittal gadolinium-enhanced T1WI demonstrates prominently enhancing nerve roots.
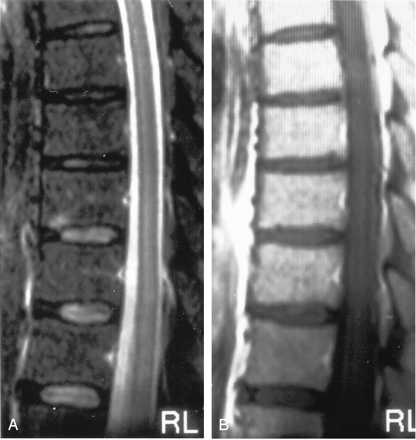
The second patient presented with bilateral lower-extremity weakness progressing to complete flaccid paralysis. Spinal T2WI revealed diffusely increased signal intensity in the conus medullaris (Fig 8A). Gadolinium-enhanced T1WI showed enhancement in the cauda equina and conus medullaris (Fig 8B). T2WI revealed demonstrated patchy involvement with increased signal intensity in the thoracic spinal cord (Fig 8C), with corresponding enhancement on gadolinium-enhanced T1WI. After 28 days of hospitalization, the patient was discharged to a long-term care facility with paraplegia and a neurogenic urinary bladder.
Another patient with intraspinal abnormalities.
A, Sagittal T2WI shows abnormal increased signal intensity in the conus medullaris.
B, Axial gadolinium-enhanced T1WI shows abnormal foci of enhancement in the conus medullaris.
C, Axial T2WI shows markedly increased signal intensity in the thoracic cord. This is appreciated despite image degradation due to artifact.
The third patient in this group presented with fever, headache, and weakness in both lower extremities. T2WI of the spine showed patchy increased signal intensity in the thoracic spinal cord (Fig 9A), with enhancement in these areas on contrast-enhanced T1WI (Fig 9B). The patient developed ascending paralysis and required endotracheal intubation leading to percutaneous tracheostomy. At the time of his transfer to the inpatient rehabilitation unit, the patient had residual weakness in all four extremities (score of 1–2 on a 5-point power assessment scale). Mechanical ventilation support was discontinued because of a gradual improvement in respiratory muscle strength. The patient was hospitalized for a total of 42 days.
Subtle thoracic cord signal abnormalities in a patient with moderate-to-severe neurologic deficits.
A, Sagittal T2WI demonstrates increased signal intensity in the mid-to-lower portion of the central thoracic cord.
B, Sagittal contrast-enhanced T1WI demonstrates patchy areas of subtle enhancement.
The Table summarizes the clinical outcomes with the different MR imaging patterns of involvement.
Clinical outcomes in different MR imaging patterns of WNV infection
Discussion
WNV is a single-stranded RNA virus. It is a Flavivirus and a member of the Japanese encephalitis virus serocomplex (7). The Culicine mosquito acts as the vector for transmitting the virus from birds to humans. Reports also describe transmission through blood transfusion (8), organ transplantation, (9) and breastfeeding (10). The incubation period in humans ranges between 3 and 14 days (7).
Most clinically important infections affect older age groups (individuals aged 50 years and older), although cases have been reported in all age groups, with no sex preference. The mortality rate is also higher in the elderly (aged 70 years and older) than in younger groups (5, 7, 11). The spectrum of clinical effects ranges from asymptomatic to fatal infections, although most infections are clinically unapparent. Fever, myalgias, headache, anorexia, generalized lymphadenopathy, and maculopapular rash can abruptly develop in one of every five infections. Myocarditis, hepatitis, and pancreatitis are rare nonneurologic manifestations of WNV infection.
Neurologic involvement is the most significant effect of WNV. The symptoms can vary from mild weakness to irreversible flaccid paralysis to a range of cognitive, sensory, and motor deficits involving the central and peripheral nervous systems. Only 35% of patients have complete recovery. The mortality rate with nervous system involvement is on the order of 10% (5, 7).
The symptoms in our patient population were comparable to those reported in other series (7, 11–13). The percentage of patients achieving complete recovery, defined as a return to baseline function, was greater in our population than in others (7, 11–13).
Our most striking observation was the presence of abnormalities on DW imaging. Diffusion restriction was seen on brain MR images in seven of 14 patients. Four of the seven patients did not have abnormalities on images obtained with any other pulse sequence. Some reports in the literature describe the presence of diffusion restriction as an early sign of viral encephalitis (14–17). This finding is followed by signal intensity abnormalities on FLAIR imaging and T2WI (14). In addition, some have noted that the diffusion abnormalities resolve before other imaging abnormalities resolve in the recovery phase (14). In our series, an important observation in the four patients with only diffusion abnormalities was the complete resolution of symptoms. No follow-up MR images were available for these patients to see if they had developed abnormalities visible on images obtained with other pulse sequences.
Diffusion restriction has been explained as a failure of adenosine triphosphate production secondary to neuronal death, as is classically seen in infarction and the associated cytotoxic edema. However, observations of reversible diffusion restriction and favorable outcomes in viral encephalitides (14, 15) and epilepsy (18) suggest that diffusion restriction need not imply cell death. Our findings, supported by those in the recent literature, at least preliminarily indicate the possibility of improved outcomes in patients with diffusion restriction without any other associated signal intensity abnormalities. In comparison, the three patients who had diffusion restriction associated with abnormal signal intensity on FLAIR images and T2WI had poor outcomes. Two of these patients died, and one had severe residual neurologic deficits; this result suggests that tissue damage has occurred when signal intensity abnormalities are apparent on FLAIR imaging and T2WI.
About 80% of the patients with normal MR images had relatively short hospitalizations (3–9 days) and recovered without any residual deficits. One patient had mild residual expressive dysphasia despite a negative brain MR image. This patient’s MR imaging study was performed 13 days after the onset of symptoms. One possible explanation could be the resolution of abnormalities during this relatively long delay. Other cases of WNV neurologic involvement with normal MR imaging findings and variable clinical outcomes are reported in the literature (19). However, the residual neurologic symptoms do appear to be less severe in these cases. To our knowledge, no statistics for prognosis are available in the literature.
In our study, hyperintensity on FLAIR imaging and T2WI in the lobar gray matter and white matter, basal ganglia, thalamus, cerebellum, and brainstem was associated with a poor outcome, including death in two of three cases. In these cases, the signal intensity abnormalities were present on FLAIR images, T2WI, T1WI and DW images. One case also had patchy enhancement on gadolinium-enhanced T1WI. The importance of this finding is uncertain at this time. The MR imaging findings, symptoms, and clinical outcomes in this group were similar to those described in a case report (20) and in two clinical series (11, 13). However, there is a paucity of images in these studies to illustrate the abnormalities.
Reports have described specific patterns of signal intensity abnormalities in the brain parenchymal structures in different viral etiologies. Bilateral involvement of the thalami and basal ganglia is discussed in case report on WNV infection (20). Similarly, past reports of Murray Valley Encephalitis and Japanese Encephalitis have described hyperintensity on T2WI of the thalami and basal ganglia (21, 22). Hyperintensity in the substantia nigra on T2WI has also been reported in St. Louis encephalitis (23, 24). These viruses, like the WNV, belong to the family Flaviviridae. Patterns of bilateral increased signal intensity on T2WI are noted in infections with other viruses, such as HIV with putaminal involvement (25), influenza virus A with bilateral thalamic abnormalities (26), and even prion-induced Creutzfeldt-Jakob disease with signal intensity abnormalities in the bilateral basal ganglia and Thalami (27, 28, 29). Although involvement in our patients was bilateral, it was not symmetrical, as reported in some of the encephalitides mentioned above (21, 25, 28). We noted no distinct pattern of WNV predilection for periventricular structures. Our patients had involvement of the cortical and deep gray matter, the cerebral and cerebellar white matter, the brainstem, and the meninges.
The meningeal involvement in two of our patients was nonspecific and can be seen with any form of meningeal inflammation. Both of these patients had severe residual neurologic deficits.
The intraspinal abnormalities seen in our study are presumably similar to those described elsewhere (11, 13), but the relative lack of images in the other studies makes direct comparison difficult. Enhancement of the nerve roots can also be seen with the subarachnoid spread of neoplasm. Similarly, increased signal intensity in the spinal cord on T2WI and enhancement on gadolinium-enhanced T1WI can be seen in any cause of myelitis. Examples include acute disseminated encephalomyelitis, multiple sclerosis, or other viral etiologies of myelitis that can lead to flaccid paralysis and the clinical picture of Guillain-Barré syndrome. However, in an appropriate clinical setting, this finding is compatible with WNV meningomyelitis (30). The prognosis for patients with these signs also was not favorable, as noted before.
An autopsy report (31) for a patient with WNV meningoencephalitis described an inflammatory infiltrate consisting of plasma cells and lymphocytes. This infiltrate was most pronounced in the midbrain, pons, and cerebellum. Involvement of basal ganglia; the temporal, parietal, and occipital lobes; and the spinal cord was less severe. The infiltrate was associated with neuronal loss, gliosis, necrosis, and edema. Lymphocytic infiltrate was also seen in the meninges and subarachnoid space. MR images in this case were grossly negative. However, the authors suggested that motion artifacts degraded the images and could have masked the abnormalities. Kelley et al (32) have reported similar findings with additional perivascular mononuclear cell infiltration. MR imaging of their case of meningoencephalitis demonstrated meningeal enhancement. Images in another patient with polio-like paralysis showed enhancement of cauda equina. One article (33) does not discuss the correlative imaging findings. Pathologic evaluation showed involvement of the brainstem and cranial nerves in the four cases reported. Our results match the areas of involvement and the symptoms mentioned in the pathology literature. Again, correlative imaging are lacking, limiting direct comparison with our cases.
Conclusion
Our study demonstrates an important trend for improved outcomes in patients without parenchymal or meningeal abnormalities on FLAIR imaging or T2WI. This group includes patients with normal brain MR images and those with abnormalities seen only on DW images. However, the number of patients was relatively small. We also defined other MR imaging patterns and the outcomes apparently associated with them. Patients with increased signal intensity in the brain and brainstem on FLAIR imaging and T2WI had the worst outcomes. Those with meningeal involvement had severe residual neurologic deficits, and patients with spinal cord and nerve root abnormalities had moderate-to-severe residual neurologic deficits.
The abnormalities that we observed are not specific for WNV and resemble findings in other meningoencephalitides and meningeal metastasis. However, unlike some viral encephalitides, WNV infection had no definite predilection for specific areas of the brain parenchyma.
References
- Received January 29, 2004.
- Accepted after revision May 10, 2004.
- Copyright © American Society of Neuroradiology