Abstract
BACKGROUND AND PURPOSE: Dissecting vertebrobasilar aneurysms are challenging to treat, and standard treatment modalities remain controversial. We retrospectively evaluated our experience using endovascular techniques to treat these aneurysms.
MATERIALS AND METHODS: From February 1997 to December 2007, 42 patients with intradural vertebrobasilar dissecting aneurysms underwent endovascular treatment. Twenty-nine patients had ruptured aneurysms, and 13 patients had unruptured dissecting aneurysms. The endovascular modalities for vertebrobasilar dissecting aneurysms were the following: 1) trapping (n = 30), 2) proximal occlusion (n = 3), 3) stent with coil (n = 6), and 4) stent alone (n = 3).
RESULTS: Seventeen of the 29 patients with ruptured vertebrobasilar dissecting aneurysms had successful outcomes without procedural complications following endovascular treatment. Procedure-related complications were the following: 1) rebleeding (n = 3), 2) posterior inferior cerebellar artery (PICA) territory infarction (n = 6), 3) brain stem infarction (n = 2), and 4) thromboembolism-related multiple infarctions (n = 1). Clinical outcomes were favorable in 32 patients (76.1%). There were 3 (7.1%) procedure-related mortalities due to rebleeding, and 1 (2.4%) non-procedure-related mortality due to pneumonia sepsis. All 13 patients with unruptured vertebrobasilar dissecting aneurysms had favorable clinical and radiologic outcomes without procedure-related complications.
CONCLUSIONS: Endovascular procedures for treatment of unruptured symptomatic dissecting aneurysms resulted in favorable outcomes. Ruptured vertebrobasilar dissecting aneurysms are associated with a high risk of periprocedural complications. Risks can be managed by using appropriate endovascular techniques according to aneurysm location, configuration, and relationship with the PICA.
Increased understanding of vertebrobasilar dissecting aneurysms, along with advances in diagnostic modalities, has resulted in such aneurysms now being regarded as more common causes of subarachnoid hemorrhage (SAH) and ischemic stroke than previously considered.1,2 Ruptured vertebrobasilar dissecting aneurysms are associated with a high incidence of rebleeding and a high mortality rate at the time of rebleeding,3–5 indicating the need for early treatment.
Trapping aneurysms by using an endovascular approach with or without bypass has been advocated as a primary treatment.6–10 There has been a gradual increase in the use of self-expandable stents for reconstructive treatment of such lesions, and current trials show favorable safety and outcome results.11,12 The natural history of unruptured symptomatic vertebrobasilar dissecting aneurysms is relatively benign,13–15 and medical treatment with anticoagulation and antiplatelet therapy appears to prevent thromboembolic complications.13–17 The indication and timing of treatment for unruptured dissecting aneurysms remains controversial.18–20 However, Naito et al21 reported that the risk of bleeding from unruptured vertebral dissections was higher than previously realized, and endovascular treatment should be considered for unruptured dissecting vertebral aneurysms susceptible to rupture.
The present study evaluated our experience using endovascular approaches for vertebrobasilar dissecting aneurysms, including unruptured dissecting aneurysms.
Materials and Methods
Between February 1997 and December 2007, 2843 aneurysms were treated by using either endovascular or surgical approaches in our institution. During this period, 47 patients with vertebrobasilar dissecting aneurysms were treated. Five of those patients underwent a direct surgical treatment and were excluded from the present analysis.
The diagnosis of a vertebrobasilar dissecting aneurysm was essentially based on the characteristic features demonstrated on conventional angiography and aided by MR or CT angiography. The clinical and radiologic data were reviewed retrospectively. Follow-up data were collected by using the most recent outpatient records. Clinical outcomes were assessed by using the Glasgow Outcome Score (GOS). Patients who returned to work with no neurologic deficits (GOS 4 or 5) were considered to have favorable outcomes. Patients with any neurologic deficit who were unable to work or required some level of assistance in their daily living (GOS 2 or 3) were considered to have poor outcomes. Radiologic outcomes were classified as follows: 1) complete or successful, or 2) incomplete or unsuccessful according to contrast filling into the distal portion of the lesion or stent deployment into the target region. Conventional angiography, MR time-of-flight angiography, or CT angiography was performed at 6–12 months to determine whether lesions had reduced in size or had healed. Additive image evaluation was performed yearly thereafter if required.
The location of aneurysms was classified into 1 of 3 groups on the basis of angiographic features: proximal to the posterior inferior cerebellar artery (PICA) (infra-PICA lesions), involving the PICA (PICA lesions), and distal to the PICA (supra-PICA lesions).
Patients with vertebrobasilar dissecting aneurysms enrolled in our study, divided into a ruptured dissecting group and an unruptured dissecting group, were evaluated and analyzed respectively. Ruptured vertebrobasilar dissecting aneurysms should invariably be treated as early as possible as a general rule in our institution. However, indications for endovascular treatment of unruptured dissecting aneurysms, all of which, except 1 case, were acute-onset (≤3 days) symptomatic lesions, were as follows: 1) pearl and string or string sign (n = 9), 2) vessel irregularity or fusiform dilation (n = 2), and 3) change of aneurysmal morphology (n = 1) (Fig. 1). One patient with an asymptomatic unruptured vertebral dissecting aneurysm underwent endovascular treatment for prevention of bleeding during major cardiovascular surgery.
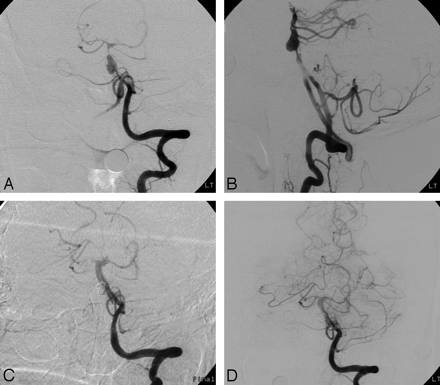
Case 5 of an unruptured vertebrobasilar dissecting aneurysm. A, Anteroposterior angiogram of the left VA shows the pearl sign with an approximately 6-mm pseudoaneurysm. B, Anteroposterior angiogram of the left VA 3 months later demonstrates marked enlargement of the pseudoaneurysm. C and D, Anteroposterior and lateral angiograms of the left VA after trapping with coils show complete occlusion of distal left VA and pseudoaneurysm.
Endovascular modalities were based on the configuration of the dissecting aneurysms and the presence of SAH. Balloon-test occlusion was not performed in our institution. Patients with supra-PICA or infra-PICA lesions were primarily considered for trapping of these lesions via detachable coil embolization. Proximal occlusion was preferentially considered for ruptured PICA lesions. Stent or stent with coil was considered for unruptured PICA lesions.
Of the 13 unruptured vertebral dissecting aneurysms, 6 were categorized as supra-PICA lesions. There were 2 aneurysms involving the PICA (PICA lesions) and 3 infra-PICA lesions. Two unruptured vertebral dissecting aneurysms could not be categorized by using conventional angiography. Two of the 3 infra-PICA lesions were treated by using stents or stent with coil, and 1, with trapping. Three of the 6 supra-PICA lesions were managed by using trapping, and the other 3 were treated by using stent or stent with coil. PICA lesions were treated with trapping and stent with coil. The reason for treating PICA lesions with trapping was that the anterior inferior cerebellar artery (AICA) supplied the PICA territory. Nondifferentiated lesions were treated by using trapping or stent with coil.
For patients with ruptured or unruptured vertebrobasilar dissecting aneurysms scheduled for stent deployment, a loading dose of dual antiplatelet medication (aspirin 300 mg plus clopidogrel 300 mg) without premedication was given immediately before the procedure.
Results
The study enrolled 42 patients with vertebrobasilar dissecting aneurysms (26 men and 16 women; ranging in age from 25 to 73 years; mean, 46.9 years) (Tables 1 and 2). Of those, 29 (69.0%) patients presented with SAH. Of the 29 patients with ruptured vertebrobasilar dissecting aneurysms, 14 aneurysms were classified as supra-PICA lesions and 7, as PICA lesions. Four ruptured dissecting aneurysms originated from the infra-PICA level (infra-PICA lesions), and 1 was a midbasilar dissecting aneurysm (Fig 2). Angiographic classification was not possible for 3 of the dissecting aneurysms. Thirteen of the 14 supra-PICA lesions were treated by using a trapping procedure, and the other was treated by using proximal occlusion. All infra-PICA lesions were treated by using trapping. Four of 7 vertebrobasilar dissecting aneurysms involving the PICA were treated by using trapping without bypass.
Treated ruptured vertebrobasilar dissecting aneurysms
Treated unruptured vertebrobasilar dissecting aneurysms
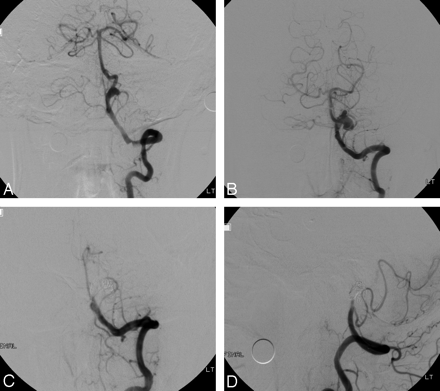
Case 14 of an ruptured vertebrobasilar dissecting aneurysm. A and B, Anteroposterior and lateral angiograms of the left VA demonstrate a pearl-and-string sign with an approximately 5-mm pseudoaneurysm in the basilar artery. C, Anteroposterior angiogram of the left VA immediately after deployment of a balloon-expandable stent into the dissected basilar lesion and occlusion of pseudoaneurysm with 4 Guglielmi detachable coils. D, Follow-up anteroposterior angiogram of the left VA 11 days later shows good patency of the basilar artery with occlusion of the pseudoaneurysm.
The reasons for trapping were the following: 1) AICA supply to the PICA territory (n = 2), 2) PICA-focal stenosis suggesting involvement of the PICA itself in dissection (n = 1), and 3) tandem lesions (n = 1). Two vertebral dissecting aneurysms involving the PICA were managed by using proximal occlusion, whereas the other lesion was treated by using stent-with-coil embolization. The midbasilar dissecting aneurysm was treated by using a balloon-expandable coronary stent with coil. All ruptured undifferentiated lesions were treated by using trapping.
All patients with unruptured vertebral dissecting aneurysms treated by using endovascular approaches showed favorable clinical outcomes (GOS 5). Of the 29 patients with SAH, 22 (75.9%) were Hunt and Hess grade II or III, and 7 (24.1%) were grade IV or V. Favorable outcomes were obtained in 19 (65.5%) of the 29 patients with ruptured vertebrobasilar dissecting aneurysms. The outcomes of the remaining 10 patients were unfavorable for 6 (20.7%) patients and death in 4 (13.8%) patients.
Complete occlusion or successful embolization was observed in all 13 patients with unruptured vertebral dissecting aneurysms. Follow-up evaluation revealed complete occlusion or successful embolization in all such patients at a mean of 21.1 months (range, 1–44 months). In ruptured vertebrobasilar dissecting aneurysms, complete occlusion or successful embolization was observed in 21 patients, and incomplete occlusion or unsuccessful embolization, in 8 patients. Follow-up evaluation was performed in 16 of 25 (64.0%) patients with ruptured dissecting aneurysms at a mean duration of 44.8 months (range, 8–114 months), and all of these patients showed complete occlusion/successful embolization or spontaneous healing. Radiologic follow-up loss occurred for 6 patients with complete occlusion/successful embolization and for 3 patients with incomplete occlusion/unsuccessful embolization.
Periprocedural complications and non-procedure-related complications were observed in patients with ruptured vertebrobasilar dissecting aneurysms. Rebleeding within 24 hours after initial endovascular treatment for vertebrobasilar dissecting aneurysms was observed in 3 patients; symptomatic PICA infarction, in 4 patients; and asymptomatic PICA infarction, in 2 patients. Symptomatic PICA infarctions were as follows: 1) ruptured vertebrobasilar dissection at the PICA level not supplied by other vascularities treated with trapping (n = 1), 2) ruptured vertebrobasilar dissection at the supra-PICA level treated with trapping with the outcome of PICA preservation seen in control angiography (n = 2), and 3) ruptured vertebrobasilar dissection at the supra-PICA level treated with proximal occlusion with the outcome of PICA preservation seen in control angiography (n = 1).
Ruptured vertebrobasilar dissection at the supra-PICA level and a nondifferentiated level treated by internal trapping resulted in asymptomatic PICA infarctions. PICA territory was supplied by the PICA itself and the AICA, seen on control angiograms. Asymptomatic brain stem infarction occurred in 2 patients. In nonprocedural complications, communicating hydrocephalus requiring a ventriculoperitoneal shunt occurred in 2 patients. Symptomatic vasospasm occurred in 1 patient; pleural effusion, in 1 patient; and sepsis, in 1 patient. All patients with rebleeding and a patient with pneumonia sepsis died. One patient with a ruptured vertebral dissecting aneurysm involving the PICA underwent proximal occlusion. Final angiography after proximal occlusion for the dissecting aneurysm revealed incomplete occlusion. The patient died of rebleeding 1 day after the procedure.
The other 2 patients with ruptured vertebral dissecting aneurysms preserving the PICA were treated with trapping. One patient with both vertebral dissecting aneurysms comprising a left ruptured dissecting aneurysm and a right unruptured dissecting aneurysm underwent trapping for the ruptured aneurysm and a stent deployment for the unruptured aneurysm. Final angiography confirmed complete occlusion of the left ruptured dissecting aneurysm and normalized patent flow for the right unruptured dissecting aneurysm. The patient suddenly developed respiratory arrest 13 hours after the endovascular procedure. Rebleeding was suspected in the left ruptured dissecting aneurysm on the basis of follow-up brain CT data. For the other patient, the final radiologic outcome showed incomplete occlusion of the dissecting aneurysm. Seizure developed and a follow-up brain CT revealed rebleeding 1 day after trapping.
Discussion
The natural history of unruptured dissecting aneurysms remains poorly understood.22 Dissection is a dynamic process, and some vertebral dissecting aneurysms may heal spontaneously, whereas others can cause ischemic symptoms or stroke due to stenosis, occlusion of the parent vessel, or involvement of perforators.22 The guidelines for endovascular treatment of unruptured dissecting aneurysms presenting with ischemia, headache, and incidental findings remain controversial. The relatively benign clinical course and outcome reported for unruptured vertebral dissecting aneurysms have resulted in conservative treatment such as anticoagulation being recommended.18–20 Anticoagulation with a target international normalized ratio of 2.0–3.0 is generally accepted for 3–6 months. However, a recent study demonstrated serial angiographic changes in 88.2% of unruptured vertebral artery (VA) dissections, including subsequent formation of aneurysms that may be amenable to surgical treatment.22 Iihara et al7 recommended short-term follow-up angiography (within 3 weeks) after presentation in unruptured vertebral dissecting aneurysms for the purpose of performing active treatment for these aneurysms. Although anticoagulation is critical for preventing thromboembolic events in the initial stages of disease, it may be insufficient to overcome the flow-limiting nature of a dissecting lesion.
A convincing argument for early neurointervention also exists.23 Naito et al21 reported that the risk of bleeding from unruptured VA dissecting aneurysms was higher than previously considered and that endovascular treatment should be considered for unruptured VA dissections associated with relatively large aneurysmal dilations or the double-lumen sign in the acute stage and with growing aneurysmal dilations during the follow-up period, on the basis of 21 patients with unruptured VA dissections.
Our rationale for treatment of unruptured dissecting aneurysm includes aneurysm morphology susceptible to bleeding and chronologic morphologic change of the VA dissection. In addition to these, our indications for endovascular treatment of unruptured dissecting aneurysms extend to acute-onset symptomatic lesions beyond typical indications for treatment. These indications based on variability of morphology in ruptured dissecting aneurysm and sudden-onset symptoms suggest the possibility of the prodromal symptoms warning of a leak. Clinical and radiologic outcomes were favorable in all patients with unruptured vertebral dissecting aneurysms treated by endovascular modalities comprising internal trapping, stents, and stent with coil. No procedural morbidity or mortality occurred in unruptured vertebrobasilar dissecting aneurysms treated with endovascular procedures. Endovascular treatment for dissecting aneurysms by using stents has been proposed, especially in patients with otherwise untreatable dissecting basilar arteries, internal carotid artery pseudoaneurysms, and dissecting VA aneurysms.24–37 Although endovascular surgery by using stents is effective for dissecting lesions, it usually does not prevent rebleeding,25 and rebleeding can occur after double-stent placement alone.9 Recently, we performed stent-only or stent-assisted coil embolization in patients with symptomatic unruptured dissecting aneurysms, without consideration of the PICA relationship. Radiologic outcomes, follow-up radiologic outcomes, and clinical outcomes were satisfactory in those patients.
Ruptured vertebrobasilar dissecting aneurysms have an extremely high rate of early rebleeding (≤70% in some series).4 Rebleeding generally occurs within the first 24 hours after rupture onset and is associated with a poor clinical outcome. Considering the aggressive behavior of ruptured vertebral dissecting aneurysms, we believe that the best and most definitive treatment is parent vessel deconstruction. This can be achieved by using either open surgical trapping or endovascular balloon or coil occlusion.6–8,32,38,39 Results of multiple series1,5,40–43 suggest that surgical or endovascular trapping to directly occlude the pathologic artery segment is appropriate in vertebral dissecting aneurysm cases.1,5,40–43 However, this strategy is not feasible for vertebral dissecting aneurysms involving the PICA not supplied by other vasculature. Proximal occlusion as an alternate strategy for trapping may allow better collateral circulation, especially when lesions involve the PICA origin. However, proximal occlusion is associated with the risk of retrograde flow into the pseudoaneurysm from the contralateral vertebral or basilar artery, with persistence of flow into the aneurysm.44 Rabinov et al8 reported rebleeding in ruptured vertebral dissecting aneurysms treated by proximal occlusion.
The present study indicates that there are considerable pitfalls associated with treating ruptured dissecting aneurysms by using endovascular treatment modalities, trapping and proximal occlusion. First, the length of the segment treated using endovascular techniques is not controllable and is generally longer than anticipated. In the case of a ruptured dissecting aneurysm in the supra-PICA level, the possibility of unwanted coil herniation into the PICA or coil packing below the PICA is always present. Second, radiologic complete occlusion is not always possible, and incomplete occlusion for ruptured dissecting aneurysms treated with trapping or proximal occlusion involves the additional risk of rebleeding. Despite these pitfalls, endovascular procedures can be regarded as a standard treatment for vertebral dissecting aneurysms due to good lesion access and relatively acceptable procedure-related complication rates.
Endovascular stent or stent with coil, the reconstructive technique for the parent vessel, has been applied to dissecting vertebral or basilar aneurysms. As limitations of parent vessel reconstruction with a stent for dissecting vertebral aneurysms, its unproven durability of treatment and the maintenance of antiplatelet medication were reported.45 However, in recent studies, outcomes of endovascular treatment by using stents appear favorable.12,31–37 In our series, outcome of that treatment was favorable as well in unruptured vertebral dissecting aneurysms. The mechanisms of stent placement for vertebral dissecting aneurysms were fundamentally based on sidewall flow models reported by Canton et al46 and histologic evidence for endothelialization of the Neuroform stent (Boston Scientific, Natick, Mass) reported by Lopes et al.47
These studies suggested that stents for dissecting aneurysms, with their radial force and metal-mesh ratio, may enhance true lumen expansion, flow diversion, and stent endothelialization to achieve aneurysm exclusion and parent artery reconstruction. However, although endovascular surgery by using stents was considered for ruptured vertebral dissecting aneurysms, heparinization and antiplatelet premedication were not performed maximally due to the possibility of rebleeding. Endovascular surgery by using stents for ruptured vertebral dissecting aneurysms requires further investigation and has, thus far, been limited to cases not treatable by using other modalities. For ruptured vertebral dissecting aneurysms that cannot be treated with trapping, combined treatment by using bypass surgery such as occipital artery (OA)-PICA anastomosis and trapping should be considered.
With respect to procedure-related complications, the present study mostly encountered rebleeding and acute PICA-territory infarction. All immediate control angiograms after endovascular procedures revealed contrast filling of the PICA. Kitanaka et al2 suggested that sacrifice of the PICA was not always dangerous because of the rich pial anastomoses. However, a retrospective review of the causes of PICA territory infarctions in the 4 supra-PICA dissecting aneurysms indicates that 1 case might have been due to a thromboembolism caused by a coil loop herniation into the PICA origin. PICA territory infarctions in the other 3 cases might have resulted from injury to a critical perforating artery supplying the brain stem and deep cerebellar nuclei or from microthromboembolism due to a coil mass exposed to the PICA.
To perform trapping for ruptured supra-PICA dissecting aneurysms and to sacrifice the PICA in cases with other poor collateral circulation covering the PICA territory, one should consider bypass techniques such as OA-PICA anastomosis to reduce the complication of symptomatic PICA territory infarction. In addition to ischemic complications of the PICA during endovascular procedures, the possibility for occluding the anterior or posterior spinal artery arising from the dissected segment should be invariably considered. Iwai et al48 reported that severe ischemic complications such as quadriparesis or respiratory disturbance could occur if the anterior spinal artery originated only from the side of the dissected VA in patients with VA aneurysms located distal to the PICA. Therefore, for planning of trapping vertebrobasilar dissecting aneurysms, these factors should be definitely considered for reducing dismal ischemic complications.
Conclusions
The present study observed favorable outcomes following endovascular approaches for symptomatic unruptured dissecting aneurysms. Before conservative management such as anticoagulation, endovascular reconstructive therapy with stent or stent with coil was feasible for a symptomatic unruptured dissecting aneurysm. However, application of these reconstructive techniques to ruptured vertebrobasilar aneurysms requires more evidence and should currently be limited to patients not treatable by using other modalities.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received January 12, 2009.
- Accepted after revision March 18, 2009.
- Copyright © American Society of Neuroradiology