Abstract
SUMMARY: Bevacizumab is a humanized monoclonal antibody that was the first FDA approved therapy designed to inhibit angiogenesis. This paper will review the mechanism of action and clinical role of this antiangiogenic agent.
Abbreviations
- FDA
- U.S. Food and Drug Administration
- VEGF
- vascular endothelial growth factor
Bevacizumab (Avastin, Genentech/Roche, South San Francisco, California) was the first US Food and Drug Administration−approved therapy designed to inhibit angiogenesis.1,2 In 2009, bevacizumab was approved for recurrent glioblastoma, and its use in early tumors is undergoing clinical trials. Before that, it had been used for treatment of various metastases. In 1989, a team of scientists isolated the human VEGF proteins, which are believed to be some of the most potent causes of angiogenesis. The oxygen and nutrient requirements of rapidly proliferating tumor cells are thought to cause the release of a hypoxia inducible factor, which in turn leads to the production of VEGF. These proteins are involved in increasing vascular permeability, inducing angiogenesis and vasculogenesis, promoting endothelial cell growth and migration, and precluding apoptosis. Because sustained angiogenesis is a hallmark of many cancers, arresting it is critical.1–4
Proposed Mechanism of Action
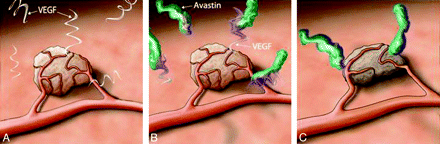
Bevacizumab is a humanized monoclonal antibody (initially it came from the mouse) that targets VEGF-A, an isoform of VEGF that stimulates endothelial cell proliferation and subsequent migration.2 Bevacizumab specifically binds to the VEGF-A protein, thereby inhibiting the process of angiogenesis (Fig 1). Studies have shown that anti-VEGF agents result in regression of existing microvessels, normalization of surviving mature vasculature, and inhibition of vessel growth and neovascularization. Maintaining the VEGF ligand inhibition may prevent tumor growth and may result in tumor shrinkage with time.1–4
Schematic Illustration of the mechanism of bevacizumab. A, There is a hypervascular tumor surrounded with VEGF protein. B, The bevacizumab compound binds to the free VEGF and reduces the concentration of the free VEGF. C, The reduction of available VEGF results in diminished blood supply to the tumor and tumor shrinkage.
Clinical Indications
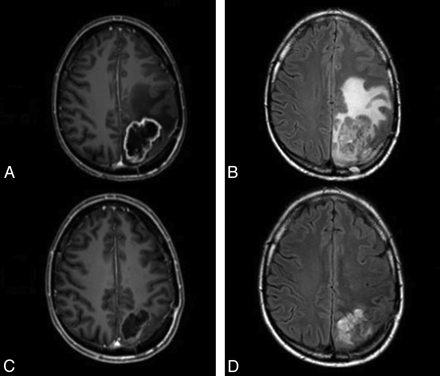
Bevacizumab has been approved for the following clinical situations: metastatic colorectal cancer, nonsquamous cell lung cancer, metastatic breast cancer, metastatic renal cell cancer, prostate cancer, and glioblastoma (Fig 2).5–12
Imaging findings associated with successful treatment with bevacizumab. A and B, Postcontrast T1-weighted (A) and fluid-attenuated inversion recovery sequences (B) show a ring-enhancing mass associated with vasogenic edema and mass effect. C and D, Following successful treatment, there is a reduction in the size of the mass and enhancement (C) and a substantial reduction in vasogenic edema (D).
Administration and Effects
Bevacizumab is a prescription-only drug administered intravenously. Its half-life is 20 days, and its metabolism route is not clear. Neurologic-related side effects include hypertension, which may lead to posterior reversible encephalopathy syndrome; hemorrhage; and nasal septum perforation.3
Economic Issues
Bevacizumab is very expensive and may not be covered by insurance. In countries with public health systems such as Canada and the United Kingdom, insurance coverage is limited. Sales of Avastin totaled 2.7 billion US dollars in 2007.4 Treatment cost per patient may be up to 100,000.00 US dollars per year, though this cost may be smaller in patients with recurrent glioblastoma due to their limited survival period.
Clinical Issues
Many of the issues described in the previous section arise because bevacizumab does not cure the underlying tumor but only extends the life span.
References
- Received November 5, 2006.
- Accepted after revision November 6, 2006.
- Copyright © American Society of Neuroradiology