Abstract
SUMMARY: Natalizumab is a humanized IgG4κ monoclonal antibody that is a selective adhesion molecule inhibitor, which prevents adhesion of leukocytes to endothelial cells. It is the first monoclonal antibody approved by the FDA for the treatment of relapsing-remitting MS. This article will review the mechanism of action and clinical role of this agent.
Abbreviations
- FDA
- US Food and Drug Administration
- FLAIR
- fluid-attenuated inversion recovery
- FSE
- fast spin-echo
- IgG
- immunoglobulin G
- MAdCAM-1
- mucosal adressin cell adhesion molecule
- MS
- multiple sclerosis
- PML
- progressive multifocal leukoencephalopathy
- TOUCH
- Tysabri Outreach Unified Commitment to Health
- VCAM -1
- vascular cell adhesion molecule-1
Natalizumab (Tysabri; Biogen Idec, Cambridge, Massachusetts and Elan Pharmaceuticals, Dublin, Ireland) was the first FDA-approved monoclonal antibody for the treatment of MS. Natalizumab received FDA approval in 2004 for treatment of relapsing-remitting MS based on the AFFIRM and SENTINEL phase 3 clinical trials.1,2 More recently, the indications for natalizumab were expanded to include Crohn disease.3–5 In 2005, natalizumab was removed from the market following cases of PML but was reintroduced by the FDA with a mandatory surveillance program in 2006 called TOUCH (Biogen).6
Proposed Mechanism of Action
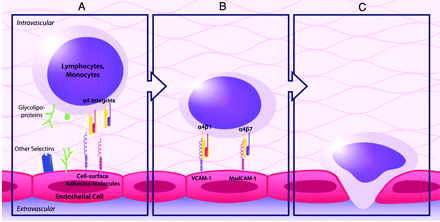
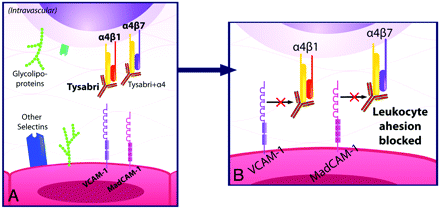
Natalizumab is a humanized monoclonal IgG4κ antibody that selectively binds to the α4-integrin component of adhesion molecules found on lymphocytes, monocytes, and eosinophils.7 α4-integrin is a subunit of the leukocyte adhesion molecules α4β1 and α4β7. In 1991, Yednock et al8 showed that targeting α4-integrin could prevent the development of demyelinating lesions in a mouse model of MS, elucidating the therapeutic potential for this medication.9 Natalizumab inhibits the interaction of α4β1 with VCAM-1 and of α4β7 with MAdCAM-1.10 VCAM-1 and MAdCAM-1 are found on endothelial cells and interact with α4β1 and α4β7 on leukocytes for firm adherence of leukocytes to endothelial cells, a requisite step for their extravasation into inflamed tissue (Fig 1).11 Natalizumab prevents migration of autoreactive leukocytes out of blood vessels into target organs by blocking the adhesion to endothelial cells of the α4-integrin component of adhesion molecules on leukocytes, inhibiting inflammation (Fig 2). Because VCAM-1 is expressed on inflamed cerebrovascular endothelial cells, α4β1 is believed to be the critical target of natalizumab in preventing leukocyte migration into the central nervous system in MS. In contrast, both VCAM-1 and MAdCAM-1 are upregulated on intestinal endothelium in Crohn disease. The efficacy of natalizumab in Crohn disease very likely is due to blockade of leukocyte adhesion factors α4β1 and α4β7 in tandem.4,7,10
The normal process of leukocyte migration out of blood vessels into tissue involves interactions between leukocytes and endothelial cells including rolling (A), adhesion (B), and extravasation (C). The adhesion molecules α4β1 and α4β7 found on leukocytes are integral in the adhesion process to endothelial cells.
A, Natalizumab blocks the adhesion of leukocytes to endothelial cells by blocking the interaction of the α4-integrin subunit of α4β1 with VCAM-1 and of α4β7 with mucosal MAdCAM-1. B, This prevents autoreactive leukocytes from exiting blood vessels and entering target organs to cause inflammation.
Clinical Indications
Natalizumab is approved for treatment of relapsing-remitting MS and Crohn disease. It is generally reserved for patients who fail first-line therapies (Figs 3 and 4).12 It must be given in conjunction with the TOUCH program, which is a national risk-minimization program designed to “minimize the risk of PML, minimize death and disability due to PML, and promote informed risk-benefit decisions regarding Natalizumab use.”6 This drug is being investigated for use in ulcerative colitis.
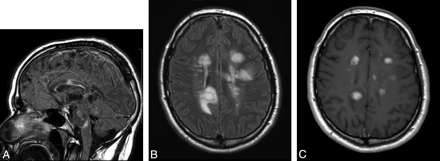
A 27-year-old man presented with numbness and weakness of both upper extremities and the left lower extremity, with multiple enhancing MR imaging lesions. He was prescribed high-dose β interferon (Rebif) soon after his initial clinical exacerbation but was switched to glatiramer acetate 1 year later due to breakthrough radiologic disease activity. The patient developed new right-sided paresthesias 3 years after his initial presentation. The MR imaging in this figure was performed when the new symptoms developed. A, FLAIR-weighted sagittal FSE image of the brain shows patchy high-signal-intensity areas involving the corpus callosum, brain stem structures, and cerebellum. B, FLAIR-weighted axial FSE image shows multiple patchy areas of high FLAIR signal intensity involving the corpus callosum and bilateral periventricular white matter with the presence of edema around a large right periatrial lesion. C, Postcontrast T1-weighted axial FSE image shows that a majority of the larger lesions exhibit intense patchy enhancement, suggestive of active demyelination.
Within 1 month of the MR imaging shown in Fig 3, the patient was started on a course of natalizumab (Tysabri), 300 mg administered intravenously every 4 weeks. Repeat MR imaging after 6 months was performed. A, FLAIR-weighted sagittal FSE image of the brain shows improvement in the patchy high-signal-intensity area of the corpus callosum with resolution of lesions involving the brain stem structures and cerebellum. B, FLAIR-weighted axial FSE image shows marked improvement in the areas of demyelination involving the corpus callosum and bilateral periventricular white matter. C, Postcontrast T1-weighted axial FSE image shows only 1 small area of enhancement in the right periventricular white matter, with lack of enhancement of the rest of the enhancing lesions.
Administration
Natalizumab is administered at specialized infusion centers enrolled in the TOUCH program for 1 hour with 1-hour monitoring. The TOUCH program also mandates regular appointments and follow-ups to monitor for signs of PML. The half-life is 11 days after 6 months of therapy.13
Side Effects
Reported serious side effects include the following:
-
Neutralizing antibody development: occurs in ≤10% of patients.1,4,5,14
-
Transfusion reaction/hypersensitivity: transfusion reactions ranging in severity from minor to anaphylaxis have been reported.1,2,12 These reactions are more common in patients with neutralizing antibodies.5
-
PML: a serious central nervous system infection due to JC virus, cases have been reported in patients with MS and Crohn disease.15 Natalizumab is recommended for use as a monotherapy in MS12 and Crohn disease to minimize risk.
-
Hepatotoxicity: clinically significant liver injury has been reported in 6 individuals without long-term liver failure.16
-
Opportunistic infection: viral encephalitis, cytomegalovirus, aspergillosis, cryptosporidium diarrhea, pneumocystis pneumonia, mycobacterium avium intracellulare, and Burkholderia cepacia pneumonia have been reported.17
-
Malignant melanoma: reports of malignant melanoma following therapy.18,19
Economic Issues
Natalizumab is given at 300 mg intravenously every 4 weeks for Crohn disease and MS. The cost is roughly $2800 per vial (300 mg) with an annual cost of $34,000. This is covered by most insurance companies.
References
- Copyright © American Society of Neuroradiology