Abstract
Summary: Endolymphatic hydrops, the primary pathologic alteration in Menière disease, can be visualized by using delayed intravenous contrast-enhanced 3D-FLAIR MR imaging. It is not known whether MR imaging–demonstrable changes of hydrops fluctuate with disease activity or are fixed. We describe the results of baseline and posttreatment MR imaging studies in a group of subjects with Menière disease with hydrops who were treated with acetazolamide. Seven subjects with untreated Menière disease with MR imaging evidence of hydrops had repeat MR imaging during acetazolamide treatment. Symptoms and imaging findings were assessed at each time point. Five subjects showed symptom improvement, of whom 3 had improvement or resolution of hydrops. One subject had recurrent symptoms with recurrent hydrops after discontinuing therapy. Two had unchanged hydrops despite symptom improvement. Subjects with unchanged symptoms had unchanged hydrops. Hydrops reversal may be seen with acetazolamide treatment in Menière disease. MR imaging may provide an additional biomarker of disease.
ABBREVIATIONS:
- hT2WI-FLAIR
- heavily T2-weighted 3D FLAIR
- MD
- Menière disease
- SPACE
- sampling perfection with application-optimized contrasts by using different flip angle evolutions
Meniere disease (MD) is an incompletely understood condition characterized by symptoms of hearing loss, aural fullness, vertigo, and tinnitus.1 The clinical phenotype and severity of symptoms vary among patients, and symptoms commonly fluctuate, typically presenting with fluctuating hearing loss that progresses and vertigo spells that eventually are associated with peripheral vestibular damage.2 A variety of treatments are available, including low-salt diet, oral diuretics, intratympanic steroid injection, and endolymphatic sac shunt surgery.3 The efficacy of these treatments has not been proved, and the mechanism of their effects is speculative.
MD is characterized by endolymphatic hydrops (ie, ballooning of the endolymphatic system), which has been described in human temporal bone postmortem studies.4,5 Recent advances in MR imaging technology have allowed in vivo imaging of endolymphatic hydrops, which has the potential to advance our understanding of the natural history of the disease and assess changes in response to treatment. Several groups have confirmed the ability of delayed intravenous contrast-enhanced MR imaging to detect hydrops,6⇓–8 but no prior studies have assessed serial changes in hydrops during diuretic treatment by using delayed intravenous contrast-enhanced MR imaging, to our knowledge. One study that followed patients treated with betahistine by using intratympanic contrast-enhanced MR imaging did not show hydrops reversal despite symptomatic improvement.9 Another study by using contrast administered through the eustachian tube showed decreased endolymphatic space size following endolymphatic sac decompression in a small group of patients.10
Our goal was to evaluate baseline and posttreatment MR imaging changes of endolymphatic hydrops in a group of patients with MD treated with oral acetazolamide, with the goal of developing a noninvasive objective biomarker for disease activity.
Materials and Methods
Subjects
This institutional review board–approved study was performed with a waiver of informed consent and a waiver of Health Insurance Portability and Accountability Act authorization. A clinical data base of 356 subjects with hearing loss and/or vestibular symptoms imaged with hydrops-protocol MR imaging (4-hour delayed double-dose intravenous contrast-enhanced MR imaging using 3D-FLAIR sequences) was queried. Seven subjects met the following inclusion criteria: 1) clinical diagnosis of definite or probable MD, as determined by American Academy of Otolaryngology–Head and Neck Surgery guidelines11; 2) pretreatment MR imaging showing evidence of endolymphatic hydrops; and 3) posttreatment MR imaging during treatment with acetazolamide. Acetazolamide treatment was initiated at a dose of 250 mg by mouth daily. Treatment was continued indefinitely if there was a response.
Imaging
MR imaging was performed on a 3T Magnetom Skyra unit (Siemens, Erlangen, Germany) by using a 16-channel head and neck coil, 4 hours following an intravenous injection of 0.2 mmol/kg of either gadopentetate dimeglumine (Magnevist; Bayer HealthCare Pharmaceuticals, Wayne, New Jersey) or gadobutrol (Gadavist; Bayer Schering Pharma, Berlin, Germany). Scanning consisted of a “cisternographic” heavily T2-weighted 3D turbo spin-echo sequence (sampling perfection with application-optimized contrasts by using different flip angle evolutions [T2 SPACE]; Siemens) and a heavily T2-weighted 3D FLAIR sequence (hT2WI-FLAIR).12 All sequences were performed as volumetric axial scans through the inner ear and internal auditory canals. The hT2WI-FLAIR sequence was performed with the following parameters: section thickness, 0.8 mm; TR/TE, 9000/534 ms; TI, 2350 ms; number of averages, 2; echo-train length, 144; flip angle, 120; matrix, 320 × 260; FOV, 200 × 167 mm; acquisition time, 6 minutes 45 seconds.
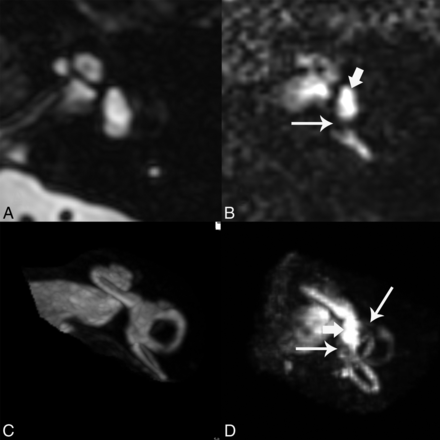
The hT2WI-FLAIR images normally show bright signal in the perilymph due to accumulation of dilute contrast in this space and dark signal in the endolymph, which remains protected from the contrast due to the presence of tight junctions. The T2 SPACE sequence shows bright signal within both endolymph and perilymph. The hT2WI-FLAIR images were reformatted in an axial plane parallel to the lateral semicircular canal to standardize interpretation and were also reconstructed as 3D maximum intensity projections. Figure 1 shows the normal appearance of T2 SPACE and hT2WI-FLAIR images. The images were evaluated with respect to the presence of endolymphatic hydrops. The qualitative criteria described by Baráth et al7 were used to assess the 2D images for the presence of hydrops in the vestibule. 3D MIPs were also reviewed, and the criteria described by Sepahdari et al8 were used to assess these images for hydrops in the vestibule. Cochlear hydrops was not specifically assessed because it was not thought that the cochlea is consistently evaluated due to the limitations of spatial resolution.
Normal appearance of cisternographic T2 (T2 SPACE) and heavily T2-weighted 3D FLAIR. A, Axial T2 SPACE source image through the midmodiolar level shows normal bright signal of fluid in the vestibule. Both endolymph and perilymph are bright by this technique. B, Axial hT2WI-FLAIR source image at the same level shows predominantly bright perilymphatic fluid in this part of the vestibule (short arrow), with a small signal void reflecting normal endolymphatic space near the ampulla of the posterior semicircular canal (long arrow). C, 3D maximum intensity projection of the T2 SPACE sequence shows the cochlea, vestibule, and semicircular canals in the same image, all with normal bright fluid signal. D, 3D MIP of the hT2WI-FLAIR sequence shows 2 small signal voids in the vestibule reflective of normal endolymphatic spaces (long arrows). The vestibule is predominantly filled with bright perilymph (short arrow).
Results
Of the 7 patients who met the inclusion criteria, all were diagnosed with definite MD. One of these patients was clinically diagnosed as having delayed endolymphatic hydrops, a subset of MD. All patients had unilateral clinical symptoms and unilateral hydrops on MR imaging. Five experienced improvement in symptoms; 3 of these 5 also showed either complete or partial reversal of hydrops (Fig 2). Two patients with symptomatic improvement did not have any change in hydrops. The 2 patients whose symptoms did not reverse showed unchanged hydrops. Clinical data are summarized in the On-line Table. Patient 3 required a dose increase to 250 mg twice daily before achieving a response. Patient 4 was only able to tolerate a dose of 62.5 mg daily due to medical comorbidities and did not have a response. Patient 1 had a return of vertigo and hydrops after attempting discontinuation of acetazolamide, and his symptoms improved after resuming treatment. A subsequent attempt to taper to 125 mg daily resulted in a return of vertigo.
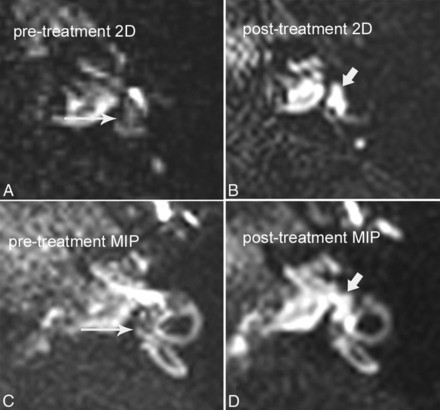
A 42-year-old man with grade I vestibular hydrops, with reversal of symptoms and reversal of hydrops after treatment with acetazolamide. A, Pretreatment axial hT2WI-FLAIR source image through the vestibule shows dilated endolymphatic spaces effacing the vestibular perilymph (long arrow). Compare with the normal appearance of the inner ear in Fig 1B. B, Posttreatment axial hT2WI-FLAIR source image through the vestibule shows interval resolution of endolymphatic hydrops, with normal bright perilymph signal in the vestibule (short arrow). C, Pretreatment hT2WI-FLAIR 3D MIP shows dilated endolymphatic spaces effacing >50% of the vestibule (long arrow). D, Posttreatment hT2WI-FLAIR 3D MIP shows interval resolution of hydrops, with normal bright perilymph (short arrow) occupying >50% of the vestibule.
The average duration of symptoms before initial MR imaging in all patients was 7.4 months. The average duration of treatment before follow-up imaging was 4.5 months. The average follow-up period was 19 months. Among the 3 patients whose symptoms and hydrops reversed, 2 continued acetazolamide treatment and were followed for an average of 9.5 months without recurrent symptoms. One patient discontinued acetazolamide and experienced recurrent symptoms with recurrent MR imaging evidence of hydrops (Fig 3). The 2 patients who had symptom improvement without hydrops reversal were imaged an average of 4 months after initiating treatment. The 3 patients with hydrops reversal were imaged an average of 6 months after treatment.
A 72-year-old man with hydrops responsive to acetazolamide and recurrence after discontinuation of treatment. A, Pretreatment axial hT2WI-FLAIR source image through the vestibule shows marked hydrops with complete effacement of the normal bright vestibular perilymphatic fluid by dilated, dark endolymphatic space (long arrow). B, Posttreatment axial hT2WI-FLAIR, during treatment with acetazolamide, shows reversal of hydrops. Normal bright perilymphatic fluid is now visible (short arrows). C, Following discontinuation of acetazolamide and recurrence of symptoms, follow-up axial hT2WI-FLAIR image shows recurrence of hydrops (long arrow).
Discussion
Delayed intravenous contrast-enhanced MR imaging is an emerging technique for evaluating patients with MD. The ability to visualize hydrops with this technique has been confirmed by multiple groups on different continents.6⇓–8,13 To date, however, no strong clinical application has been established for this technique, and existing research is mostly limited to showing a connection between a clinical diagnosis of MD and imaging evidence of hydrops.
By showing reversibility of hydrops, we demonstrate that MR imaging can provide a biomarker of disease. This is critical for evaluating the effects of various treatments, particularly when exploring new therapies. Symptoms are known to fluctuate in MD and hence do not provide a sufficient marker of treatment effectiveness on their own. Audiometric testing and vestibular evoked myogenic potentials offer added objective measures of disease severity in MD but may also be confounded by other variables, such as presbyacusis or additional pathology such as otosclerosis.14 MR imaging visualization of hydrops provides a unique, quantifiable marker of a direct effect of the disease.
One patient experienced reversal of symptoms and reversal of MR imaging–evident hydrops while on acetazolamide and re-emergence of symptoms with re-emergence of hydrops after stopping diuretics. Although this was just a single case, it shows a promising connection among acetazolamide treatment, symptoms, and hydrops.
Notably, 2 of 5 patients in our study who had symptom reversal did not have hydrops reversal. The reasons for this result are unclear because the small number of subjects does not permit a robust statistical analysis. However, factors such as the duration of disease before treatment and the length of time between treatment and repeat imaging may influence the results. Alternatively, the symptomatic improvement in these patients may have been a coincidence related to normal symptom fluctuation rather than an effect of the treatment. Rather than considering this phenomenon as a limitation of MR imaging, we view it as evidence that MR imaging provides unique information that is not duplicated by eliciting a clinical history from the patient.
The primary limitation of this study is the small number of subjects. Although a high volume of hydrops-protocol MRI is performed at our institution, only a small number of subjects met the inclusion criteria for this analysis. Despite the small number of subjects, the results provide support for the use of pre- and posttreatment hydrops-protocol MR imaging in prospective investigations of treatments for MD. There are also inherent technical limitations of the MR imaging technique and limits of image interpretation. Although our MR imaging technique has equal or superior resolution to other techniques reported in the literature, further technical improvements are needed to confidently visualize the entire membranous labyrinth. The method of image assessment, categorizing each ear as normal, grade I hydrops, or grade II hydrops, may be insensitive to subtle changes among scans. Further advancements in image acquisition and image interpretation techniques are needed to fully realize the potential of hydrops imaging.
Conclusions
Pilot data from a group of patients imaged with hydrops-protocol MR imaging before and during acetazolamide treatment show that endolymphatic hydrops is a reversible feature of Menière disease. Hydrops-protocol MR imaging provides a unique biomarker of disease in MD and may be valuable in assessing the effects of treatment.
Footnotes
Drs Vorasubin and Sepahdari contributed equally to this work.
Preliminary results of this work were previously presented at: Annual Meeting of the American Otological Society, May 16–17, 2014; Las Vegas, Nevada.
References
- Received March 9, 2015.
- Accepted after revision May 25, 2015.
- © 2016 by American Journal of Neuroradiology