Abstract
BACKGROUND AND PURPOSE: Surgical excision of an affected sublingual gland for treatment of a ranula can carry a potential of a nerve damage or postoperative complications. However, there have been little studies about effective minimally invasive therapeutic method, yet. Our aim was to evaluate the efficacy and safety of ethanol ablation of ranulas and the clinicoradiologic factors that can predict outcome.
MATERIALS AND METHODS: This retrospective study evaluated 23 patients with ranulas treated by percutaneous ethanol ablation. Treatment outcome was assessed in 20 patients followed for at least 6 months. The duration of symptoms before ethanol ablation, pretreatment volume, and parapharyngeal extension on sonography and/or CT were correlated with the outcome. The Mann-Whitney U test and Fisher exact test were used for comparison of the factors according to the outcome.
RESULTS: The study evaluated 14 males and 9 females with a median age of 26 years (range, 3–41 years). Among 20 patients who were followed for at least 6 months (median, 20 months; range, 6–73 months), 9 patients (45%) demonstrated complete disappearance of the ranulas and 11 (55%) showed an incomplete response. When the patients were divided according to the duration of symptoms before ethanol ablation, the complete response rate was significantly higher in patients with ≤12 months of symptoms (73%, 8/11) than that in others (11%, 1/9) (P = .010). Pretreatment volume and parapharyngeal extension were not significantly different between the 2 groups.
CONCLUSIONS: Ethanol ablation is a safe and noninvasive treatment technique for ranulas with a significantly better outcome in patients with ≤12 months of symptoms. Therefore, it could be considered an alternative nonsurgical approach for ranulas with recent onset of symptoms.
ABBREVIATION:
- EA
- ethanol ablation
A ranula is a pseudocyst or mucous-retention cyst that arises from leakage of saliva from the sublingual or minor salivary gland.1 Ranulas have traditionally been surgically treated by excision of the affected sublingual gland with or without the excision of the ranula.1,2 Surgical excision is a definitive treatment with very low recurrence rates, ranging from 0% to 2%.3,4 However, excision of the sublingual gland can be technically difficult and carries a potential risk of damage to the surrounding vital structures, including the lingual nerve and the submandibular duct, with postoperative complication rates ranging from 11% to 29%.3,4 Therefore, there is a need for a nonsurgical minimally invasive treatment of ranulas.
Among chemical ablation agents, picibanil (OK-432; Chugai Pharmaceutical Co, Tokyo, Japan) has been most commonly reported for minimally invasive treatment of ranulas.1,3,5⇓⇓⇓–9 Intracystic injection of OK-432 is safe without serious complications, but the recurrence rate is relatively high, from 23% to 48%.1,3,8 Therefore, more effective sclerosing agents are necessary for successful minimally invasive treatment of ranulas.
Ethanol is an effective sclerosing agent2,10; its effects include instantaneous cellular dehydration and protein denaturation that result in the clumping of blood cells and vessel wall necrosis, followed by thrombosis and occlusion of vessels.10,11 It has been reported that ethanol ablation (EA) is effective and safe for the treatment of benign cervical cystic lesions, including cystic thyroid nodules, thyroglossal duct cysts, and lymphatic malformations.12⇓⇓⇓⇓⇓–18
To the best of our knowledge, no previous studies have examined the treatment efficacy and safety of EA for ranulas. The purpose of this study was to evaluate these features and the clinicoradiologic factors that can predict its outcome in a retrospective cohort.
Materials and Methods
Patients
This study was approved by the institutional review board of our institution (University of Ulsan College of Medicine, Asan Medical Center), and written informed consent for EA was obtained from all patients before the procedure. We searched the electronic medical records from 2010 to 2015 to find consecutive patients treated with EA after confirmation of a ranula by aspiration of thick mucus fluid.
Preprocedural Evaluation
In addition to basic demographic data, we also evaluated the duration of symptoms before EA and the history of prior treatment. In all patients, the preprocedural sonography was performed by 1 radiologist (J.H.L., with 14 years of experience in performing sonography-guided procedures of the head and neck regions) with 1 of 2 sonographic systems: an Acuson S3000 Ultrasound System (Siemens, Erlangen, Germany) or an EUB-7500 unit (Hitachi Medical Systems, Tokyo, Japan) equipped with a linear, high-frequency probe (6–18 or 5–14 MHz). The sonographic examination was performed to determine the location of the ranula, the presence of direct contact with the sublingual gland, and the volume of the cystic lesion. If available, contrast-enhanced CT was also evaluated for the aforementioned imaging findings.
Ethanol Ablation and Follow-Up
All EAs were performed by the same radiologist (J.H.L), with the patient in a supine position and the neck extended under local anesthesia with 2% lidocaine. Moderate sedation was used for an uncooperative child. A 16- to 18-gauge needle was inserted into the ranula to aspirate the thick mucus fluid with a 30-mL syringe via a short connector. Internal debris and residual thick mucus material were cleared by irrigation with saline. Before the ethanol injection, 2% lidocaine was injected and retained inside the pseudocyst for 30 seconds to check for any sensory or motor changes induced by the lidocaine anesthesia and for pain control. After re-aspiration of the lidocaine, 100% ethanol was slowly injected into the ranula. The amount injected was determined to be approximately 50% of the volume of the aspirate. The injected ethanol was completely re-aspirated after remaining in the ranula for 10 minutes.
Patients were re-evaluated at 1-, 6-, and 12-month intervals after EA and then annually with sonography and/or CT examination to check for the recurrence of the lesion or of symptoms. EA was reperformed if there was a cystic cavity of >50% of the initial volume at the 1-month follow-up or if there was a recurrence of a cystic lesion with an estimated volume of >1–2 mL during the remainder of the follow-up period. However, none of the patients were treated with EA >3 times.
Statistical Analysis
The final treatment outcome was assessed at the last follow-up as a complete response (complete disappearance of the cystic cavity), an incomplete response (decreased volume of the cystic cavity), or no response (unchanged cystic cavity). Treatment response was only assessed in patients who were followed up for at least for 6 months after the last EA. We tested for an association between the final outcome and the duration of symptoms before EA, pretreatment volume, and extension to the parapharyngeal space on sonography or CT with a Mann-Whitney U test and Fisher exact test.
All statistical analyses were performed with the SPSS, Version 12.0 (IBM, Armonk, New York). The Mann-Whitney U test and Fisher exact test were used for comparison of the factors according to the outcome. A P value < .05 was considered statistically significant.
Results
Twenty-three patients were treated with EA, including 14 males and 9 females with a median age of 26 years (range, 3–41 years). Among the 23 patients, 19 had a plunging ranula continuous with a herniated sublingual gland in the submandibular space, 2 had simple ranulas, and 2 had ranulas with direct contact with the submandibular gland instead of the sublingual gland.
Seven of the 23 patients had a history of prior treatment, including 5 patients who had been treated with chemical ablation with OK-432 and 2 patients who had been surgically treated (one via intraoral excision of the ranula and the other via excision of the submandibular gland). The number of EA sessions was 1 in 11 patients, 2 in 10 patients, and 3 in 2 patients. There were no procedure-related complications in any patients.
Twenty of the 23 patients were followed up for at least 6 months after EA and were assessed for the final treatment outcome at the last follow-up. The median follow-up time after the last EA was 20 months (range, 6–73 months). Nine of 20 (45%) patients demonstrated complete disappearance of the ranula (Fig 1), but 11 (55%) demonstrated an incomplete response (Fig 2). Four of 11 patients with an incomplete response underwent surgical excision of the sublingual gland after recurrence. The clinicoradiologic characteristics and outcome of all patients are summarized in the Table.
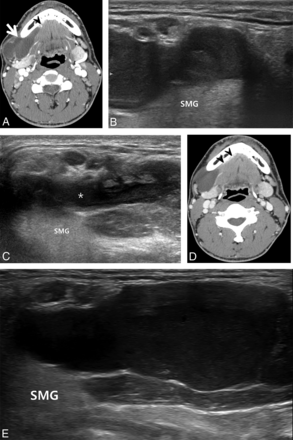
A 19-year-old man with a plunging ranula in the right submandibular space developed 1 month previously. A and B, Pretreatment contrast-enhanced axial CT images. A, Axial CT image shows a well-defined cystic mass in the right submandibular (thick arrow) space. B, Another CT image demonstrates the cystic mass continuing into the sublingual space (thin arrow), showing direct contact with bilateral sublingual glands (arrowheads). C, Pretreatment oblique sonogram shows a fluid collection with internal debris in the right submandibular space (arrows). D, Sonogram 1 month after 2 sessions of ethanol ablation in the same plane as C shows complete disappearance of the ranula in the submandibular space. E, A sonogram 12 months after D in the same plane shows complete disappearance of the ranula in the submandibular and sublingual spaces. SMG indicates submandibular gland; M, mylohyoid muscle; D, anterior belly of the digastric muscle.
A 32-year-old man with a plunging ranula in the right submandibular space for the past 16 months. A, Pretreatment contrast-enhanced axial CT image shows a well-defined lobulated cystic mass (arrow) in the right submandibular space showing direct contact with the herniated right sublingual gland (arrowhead). B, Pretreatment oblique sonogram shows a fluid collection in the right submandibular space. C, Sonogram 1 month after ethanol ablation demonstrates the remaining fluid in the same space (asterisk). D, Contrast-enhanced axial CT image 34 months after ethanol ablation shows the cystic mass in the right submandibular space, adjacent to the herniated right sublingual gland (arrowheads). E, Oblique sonogram 34 months after ethanol ablation also demonstrates a cystic mass in the same space, showing an increase in size compared with C. SMG indicates submandibular gland.
Summary of clinicoradiologic factors and outcome after ethanol ablation
Among the 9 patients with complete response, EA was performed once in 7 patients and twice in 2 patients. Both patients with simple ranulas showed a complete response after a single session of EA. Among the 11 patients with incomplete responses, the number of EA sessions was 1 in 2 patients, 2 in 7 patients, and 3 in 2 patients, which was significantly different from that in those with complete responses (P = .026).
When the clinical and radiologic factors were compared between the complete and incomplete response groups, the duration of symptoms before EA was significantly shorter in the complete response group (median, 2 months; range, 1–12 months) than in the incomplete response group (median, 13 months; range, 2–240 months; P = .001). When the patients were divided according to the duration of symptoms, the complete response rate was significantly higher in patients with ≤12 months of symptoms (73%, 8/11) than in those with >12 months of symptoms (11%, 1/9) (P = .010). The complete response rate was not significantly different between pediatric (3/5, 60%) and nonpediatric (6/15, 40%) patients (P > .05). The other factors, including pretreatment volume and the presence of a parapharyngeal extension, were not significantly different between the complete (median, 9 mL; range, 3–30 mL; n = 4, 44%) and the incomplete (median, 9 mL; range, 3–45 mL; n = 4, 36%) response groups (P > .05).
Discussion
In this study, we demonstrated that EA could be safely performed without any adverse events and could achieve complete disappearance of ranulas in 45% of patients at the 6-month follow-up. We also found that EA was significantly more effective and had a significantly higher complete response rate in patients with ≤12 months of symptoms, compared with those with a longer duration of symptoms (73% versus 11%, respectively). Although long-term follow-up results are still unknown, EA could be an alternative nonsurgical approach for patients experiencing symptoms for ≤12 months, given the potential risk of damage to the lingual nerve and the submandibular duct in an operation.
Two studies have previously reported a complete response rate of 52%–77%1,8 after chemical ablation of ranulas with OK-432 at a median follow-up of 10–12 months. The studies reported adverse events such as early rupture of the ranula (37%), fever lasting 2–3 days after injection (57%), injection site swelling for 1 week (47%), mild odynophagia (33%), and 1 incident of severe swallowing difficulty, which required admission for treatment.1,8 Neither of the previous studies correlated the clinical or radiologic characteristics with the treatment outcome or reported the pretreatment duration of symptoms among the enrolled patients.
OK-432 is a lyophilized mixture of a low-virulence strain of group-A Streptococcus pyogenes incubated with benzylpenicillin.2 The sclerosing effect of OK-432 retained in the cystic cavity is due to a strong local inflammatory reaction by activated neutrophils and monocytes, which secondarily produce cytokines and induce transient flulike symptoms or early rupture of the treated ranula. In addition, the use of OK-432 is contraindicated in those with penicillin allergy.5,8 On the other hand, ethanol acts as a sclerosing agent by instantaneous dehydration of the cyst wall, protein denaturation, clumping of blood cells, and vessel wall necrosis,2 which allow us to re-aspirate the injected ethanol after 5–10 minutes of retention. We believe that re-aspiration of the ethanol may reduce the potential for adverse events, such as facial flushing or dizziness, due to systemic absorption of the chemical ablation agent or site swelling or odynophagia from leakage at the injection site.
Another technique that may minimize local complications is the injection of lidocaine into the ranula after aspiration of the thick mucus fluid. We used 2% lidocaine at half the volume of the aspirate for 30 seconds before the ethanol injection in all cases, first, to check for the presence of the hypoglossal or the lingual nerve and, second, to locally anesthetize the surrounding soft tissues. No motor or sensory changes were noted in any of the patients after lidocaine injection in this study.
To our knowledge, this is the first report to evaluate the efficacy of EA in the treatment of ranulas. Although the overall complete response rate at 6-month follow-up was lower than anticipated, we were able to determine that the duration of symptoms was the only clinicoradiologic factor predicting a better outcome after EA in patients with ranulas. Neither the pretreatment volume nor the parapharyngeal extension was associated with the treatment outcome. Given that ductal disruption by trauma is a major causative factor of ranulas, persistent leakage of mucus fluid might hinder the sealing off of the leaking point with ethanol.
This study has several limitations in addition to its retrospective nature. First, we did not evaluate the long-term follow-up outcome after EA. However, we demonstrated the safety of EA for treating ranulas and described some techniques that may improve its safety. We believe our results could be the basis of promoting further studies with larger groups of patients and longer follow-up periods. Second, our study included 7 patients with a history of prior treatment, including surgery or chemical ablation with OK-432. Because the number of enrolled patients was small, we could not evaluate the potential confounding effects of previous treatments. This should be further investigated in future studies. Last, the sample size of this study was too small to generalize our results, which should be proved with further larger studies.
Conclusions
EA is a safe noninvasive treatment technique for ranulas, showing significantly better outcomes in patients with ≤12 months of symptoms. Given the potential risk of damage to the lingual nerve and the submandibular duct during an operation, EA could be considered an alternative nonsurgical approach for patients with symptoms for ≤12 months.
References
- Received December 27, 2016.
- Accepted after revision May 9, 2017.
- © 2017 by American Journal of Neuroradiology