Abstract
SUMMARY: Myalgia is a previously reported symptom in patients with COVID-19 infection; however, the presence of paraspinal myositis has not been previously reported. We report MR imaging findings of the spine obtained in a cohort of 9 patients with COVID-19 infection who presented to our hospital between March 3, 2020 and May 6, 2020. We found that 7 of 9 COVID-19 patients (78%) who underwent MR imaging of the spine had MR imaging evidence of paraspinal myositis, characterized by intramuscular edema and/or enhancement. Five of these 7 patients had a prolonged hospital course (greater than 25 days). Our knowledge of the imaging manifestations of COVID-19 infection is expanding. It is important for clinicians>a to be aware of the relatively high frequency of paraspinal myositis in this small cohort of patients with COVID-19 infection.
ABBREVIATIONS:
- COVID-19
- coronavirus disease 2019
- SARS-CoV-2
- Severe Acute Respiratory Syndrome coronavirus 2
Information regarding the imaging manifestations in patients infected with Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2) has been rapidly evolving; however, most of the imaging studies have primarily focused on the pulmonary, gastrointestinal, and cardiac manifestations of coronavirus disease 2019 (COVID-19).1⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓-15 Emerging case reports have described neuroimaging manifestations seen in patients with COVID-19 infection, including acute intracranial hemorrhage, demyelinating lesions, and encephalitis.16
The presence of paraspinal muscular pathology in the setting of COVID-19 infection has not been previously reported. Although the most common symptoms of COVID-19 infection include cough, shortness of breath, and fever, myalgia has been reported in up to 50% of patients with the infection.17-18 The purpose of this study was to describe the imaging findings of suspected myositis in a small cohort of patients with COVID-19 infection undergoing MR imaging of the spine.
CASE SERIES
This study was an institutional review board–approved, retrospective study performed at a single large academic institution. Between March 3, 2020 and May 6, 2020, 641 patients presented to our institution for medical care and tested positive for COVID-19 by reverse transcriptase polymerase chain reaction. A total of 9 patients with COVID-19 infection at our institution underwent MR imaging of the spine for evaluation of spinal pathology with indications including back pain, lower extremity weakness, and lower extremity paresthesia.
All imaging was performed on either a 1.5T (Signa HX and Signa Excite HDx, GE Healthcare; Avanto, Siemens) or 3T scanner (Discovery MR750, GE Healthcare; Tim Trio and Skyra, Siemens). All MR imaging examinations of the spine included sagittal T1-weighted imaging, sagittal non-fat-saturated T2-weighted imaging, sagittal fat-saturated T2-weighted imaging, and axial T2-weighted imaging. Postcontrast sequences were performed in a subset of this cohort.
Evaluation of the MR imaging included an assessment for spinal cord signal abnormality and clumping or thickening of the cauda equina nerve roots, bone marrow edema suggestive of an acute process, edema and/or enhancement within the intervertebral disks, and edema and/or enhancement within the paraspinal musculature. Myositis was defined as intramuscular edema manifested by T2 hyperintensity and/or enhancement within the paraspinal muscles which was present in the absence of, or disproportionate to the presence of minimal edema in the posterior subcutaneous soft tissues. The locations of these findings were recorded in reference to the vertebral body level for each patient. Indications for spine MR imaging included a report of back pain in 5 patients, bilateral leg pain in 2 patients, imbalance in 1 patient, and 1 patient who was found unconscious after a suicide attempt.
A complete cervical, thoracic, and lumbar spine MR imaging was performed in 3 patients, an isolated cervical spine MR imaging was performed in 1 patient, thoracolumbar MR imaging was performed in 3 patients, and isolated lumbar spine MR imaging was performed in 2 patients (On-line Table). Four of the 9 MR imaging examinations were performed with contrast and the remaining 5 MRIs were performed without contrast.
Prior MR imaging of the spine was available for 3 patients in this cohort confirming that no findings of myositis were seen on prior spine MR imaging. Additionally, the electronic medical records were searched for each patient and confirmed that none of these patients had been previously treated for myositis. One patient in this cohort had a single-level laminectomy in the thoracic spine for treatment of an epidural abscess 5 years earlier. No history of prior spine surgery was present in the remaining 8 patients.
Imaging findings were correlated with clinical parameters. Patient age, sex, length of hospital stay, respiratory failure requiring intubation, presence of a superimposed bacterial infection, and serum inflammatory markers were recorded for each patient. The recorded serum inflammatory markers included creatine kinase levels (normal range 60–400 U/L), erythrocyte sedimentation rate (normal range 0–20 mm/h), C-reactive protein (normal range <8.0 mg/L), creatinine level (normal range 0.6–1.5 mg/dL), and D-dimer (normal range <500 mg/mL). All serum inflammatory markers recorded were performed within 12 hours of the MR imaging examination.
Of the 9 patients included in this cohort, the mean age was 55 years (standard deviation 17 years, range: 30–87). Six of the 9 patients required admission to the hospital for treatment of COVID-19 infection, and 4 of these patients were intubated. The mean time between hospital admission and/or symptom onset and acquisition of the MR imaging scan was 17 days (range 1–35 days following admission). None of the patients in this cohort were diagnosed with having a superimposed bacterial infection over the duration of their hospital courses.
Seven of 9 (78%) patients who underwent spine MR imaging demonstrated evidence of myositis on MR imaging with involvement of the erector spinae muscles and multifidus muscles (Figs 1⇓–3). In all 7 patients with MR imaging findings of myositis, it occurred exclusively in the lumbar spine and involved multiple vertebral body levels (On-line Table). In all cases, the myositis was bilateral.
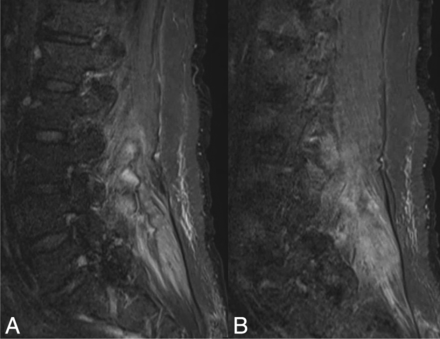
A 56-year-old man with COVID-19. A, sagittal fat-saturated T2-weighted image of the lumbar spine demonstrating increased T2 signal intensity within the posterior paraspinal muscles. B, postcontrast fat-saturated T1-weighted image of the lumbar spine demonstrating diffuse enhancement within the posterior paraspinal muscles.
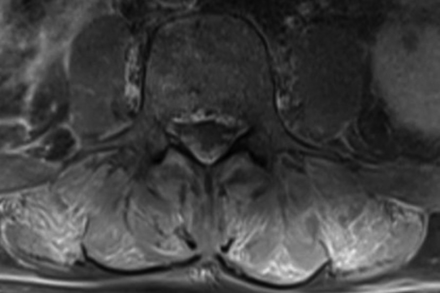
Same 56-year-old man with COVID-19 seen in Fig 1. Axial postcontrast, fat-saturated T1-weighted image of the lumbar spine demonstrates symmetric areas of intramuscular enhancement involving the erector spinae and multifidus muscles bilaterally.
A 54-year-old woman with COVID-19. Sagittal fat-saturated T2-weighted imaging of the lumbar spine demonstrates nearly symmetric areas of increased T2 signal intensity within the posterior paraspinal muscles bilaterally. A, right parasagittal view of the T2 signal hyperintensity in the right paraspinal musculature. B, left parasagittal view of the T2 signal hyperintensity in the left paraspinal musculature.
All patients with lumbar spine imaging (8 total) had trace to mild edema within the superficial subcutaneous soft tissues. In the 7 patients with lumbar spine MR imaging who had imaging findings suggestive of myositis, the edema in the paraspinal musculature was out of proportion to the degree of superficial subcutaneous edema.
None of the patients in this cohort demonstrated signal abnormality or enhancement within the visualized portion of the spinal cord or clumping or thickening of the cauda equina nerve roots. Abnormal edema and/or enhancement within the intervertebral disks was not seen in any patient in this cohort. Two of the 9 patients in this cohort had degenerative changes related to moderate facet hypertrophy confined to 2 levels within the lumbar spine. There was no evidence of reactive marrow edema in these patients related to degenerative disease.
One patient included in this cohort had a history of adrenal carcinoma with a known metastatic lesion at the L5 level, which was unchanged in appearance from multiple prior MR imaging examinations, and was not associated with new marrow edema or extension of disease.
Of the 7 patients with MR evidence of myositis, 5 were admitted to the hospital with a length of stay greater than 25 days, and 4 of these 5 patients were intubated over the course of their hospital admission. Three of the 7 patients with myositis demonstrated elevated serum inflammatory markers (as shown in the On-line Table). MR findings suggestive of myositis were seen in 2 patients who did not require hospital admission, did not have symptoms of respiratory failure, and did not have serum inflammatory markers collected.
None of the patients in this cohort received hydroxychloroquine or remdesivir for treatment of COVID-19 infection.
DISCUSSION
Of the small cohort of patients with COVID-19 infection who underwent MR imaging at our institution for evaluation of the spine, 78% (7 of 9) of patients had intramuscular edema and imaging findings suggestive of myositis, and all cases of myositis were found in the lumbar spine. These findings occurred in the absence of spine trauma. Three of the 7 patients in this cohort with MR imaging findings suggestive of myositis had elevated inflammatory markers. Two patients in our cohort did not demonstrate imaging manifestations of lumbar myositis; 1 had a relatively short hospital admission of 10 days, and the other patient was asymptomatic and did not require hospital admission. The patients in this cohort who had imaging of the cervical and thoracic spine demonstrated no MR imaging findings to suggest myositis in these segments of the spine.
Possible etiologies of the observed paraspinal myositis include direct muscular viral infection with SARS-CoV-2, an immune-mediated, parainfectious inflammatory response given the elevated serum inflammatory markers in several patients, or drug-mediated effects. An additional consideration for these findings includes sequelae of a critical illness myopathy that has been previously described in up to 62% of intensive care unit patients who failed to be wean from the ventilator.19-20 While it is possible that these findings could be compounded by third spacing with gravitationally dependent edema, this is felt to be less likely as edema and/or paraspinal muscular enhancement was found to be out of proportion to the degree of overlying superficial subcutaneous edema, which was found to be trace to mild in all patients. Of the 7 patients with MR imaging findings of myositis, 3 patients were never intubated, and 4 patients had MR imaging findings suggestive of myositis with MR imaging performed within 10 days of admission. These findings suggest that patients may have been relatively early in their COVID-19 infection course and these findings may be unrelated to a critical illness myopathy. Viral myositis is a known entity reportedly caused by several other viruses including influenza, HIV, hepatitis C, and Middle East Respiratory Syndrome Coronavirus.21⇓⇓⇓-25 The results of this study are consistent with Beydon et al19 who demonstrated MR features of myositis involving the pelvis and thighs in a patient with COVID-19 infection.
This is a small series of patients, in part related to the low frequency of back pain complaints in patients with COVID-19 infection. Furthermore, severe manifestations of COVID-19 may render patients too unstable or unable to undergo MR imaging of the spine. There are numerous confounders in these patients including heterogeneous sampling of serum inflammatory markers, lack of clear information regarding how long patients had the infection before experiencing symptoms of back pain and weakness, and heterogeneous treatment algorithms. These variations in practice patterns, documentation, and recorded histories make it difficult to determine dominant etiologies contributing to these imaging manifestations.
In conclusion, the findings of this study demonstrate a high frequency of myositis in patients with COVID-19 infection reporting myalgia in a small series. It is important for health care providers and radiologists to be aware of these clinical features and imaging findings.
Footnotes
Disclosures: William A. Mehan—UNRELATED: Consultancy: Kura Oncology, Comments: Independent reviewer of head and neck imaging studies for a clinical trial; Expert testimony: CRICO and other health insurance companies, Comments: Expert opinion for medicolegal cases involving neuroimaging.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received May 26, 2020.
- Accepted after revision June 12, 2020.
- © 2020 by American Journal of Neuroradiology