Abstract
SUMMARY: Coronavirus disease 2019 (COVID-19) is associated with a severe inflammatory response. Inflammation affects atherosclerotic plaque vulnerability and promotes a thrombogenic environment. We report a series of 6 patients with COVID-19 with acute ischemic stroke due to intraluminal carotid artery thrombus presenting during an 8-day period. Six patients were included (5 men) with a mean age of 65.8 years (range, 55–78 years). COVID-19 was diagnosed by detection of Severe Acute Respiratory Syndrome coronavirus 2 in 5 patients and was presumed due to typical clinical and imaging findings in 1 patient. All patients had vascular risk factors including diabetes (83%), hyperlipidemia (100%), and smoking (17%). Four patients presented with large infarcts with initial NIHSS scores of 24–30. During their hospitalization, all patients had elevated D-dimer and C-reactive protein levels, 5 patients had elevated lactate dehydrogenase and ferritin levels, 3 had elevated interleukin-6 levels, and 2 had elevated troponin levels. Inflammation related to COVID-19 may result in rupture of vulnerable atherosclerotic plaques, resulting in thrombosis and acute ischemic stroke.
ABBREVIATIONS:
- COVID-19
- coronavirus disease 2019
- ICT
- intraluminal carotid artery thrombus
- IL-6
- interleukin-6
- LVO
- large-vessel occlusion
- SARS-CoV-2
- Severe Acute Respiratory Syndrome coronavirus 2
Since emerging in late 2019, the Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2) and associated coronavirus disease 2019 (COVID-19) have rapidly progressed to a global pandemic. Current understanding suggests that COVID-19 causes an excessive immune response, resulting in inflammation and extensive tissue damage.1 Those patients who develop severe disease have been found to have significantly elevated levels of interleukin-6 (IL-6) and other inflammatory cytokines in what is termed a “cytokine storm.”2 This pattern of severe inflammation is of concern in the realm of vascular neurology because previous coronavirus and influenza epidemics have demonstrated an increased risk of cardiovascular complications.3 These findings were attributed primarily to the substantial inflammatory response along with localized vascular inflammation.3,4 More recent studies have also found that flulike illnesses increase the odds of cerebral infarction by nearly 3-fold during the initial 15 days, with inflammation again identified as a potential culprit.5
A specific subtype of acute ischemic stroke, large-vessel occlusion (LVO), is characterized by occlusion of a major extracranial (carotid or vertebral artery) or intracranial vessel and represents 24%–38% of all acute ischemic strokes when defined as blockage of the intracranial ICA, M1, M2, A1, vertebral artery, P1, or basilar artery.6 One important cause of LVO is artery-to-artery embolism, usually due to the presence of an atherothrombotic plaque or thrombosis at the site of a plaque rupture. Of particular concern are atherosclerotic plaques that are considered vulnerable, meaning those that have a high probability of undergoing rupture and causing local thrombosis and embolism. Plaque vulnerability is determined, in part, by plaque morphology, which is influenced by pathophysiologic mechanisms occurring at the cellular and molecular levels.7 One factor that has been reported to have a key role in promoting plaque vulnerability is inflammation, which can lead to thinning of the fibrous caps, enhanced influx of lipids and expansion of the lipid core, as well as increased neoangiogenesis.8
Cerebral infarction related to supracardiac atherosclerosis and subsequent thromboembolism is an increasingly recognized cause of embolic stroke.9 A 2019 meta-analysis found atherosclerotic plaques with high-risk features to be 5 times more prevalent in the ipsilateral compared with the contralateral carotid artery in embolic stroke of underdetermined source.10 Nevertheless, identification of intraluminal carotid artery thrombus (ICT) is less common. In the North American Symptomatic Carotid Endarterectomy Trial (NASCET),11 the frequency of ICT was only 1.1% in symptomatic patients with <70% stenosis versus 4.3% and 5.5% in patients with ≥70% and >85% stenosis, respectively.12 While ICT can occur in patients with and without significant atherosclerotic disease, in patients without underlying carotid stenosis, ICT is often associated with a hypercoagulable state.12
In the context of the current pandemic, initial reports have indicated that fewer patients are seeking emergency care for stroke symptoms, possibly to avoid exposure to the coronavirus. Despite an overall reduction in our total institutional stroke volumes, we noted an unusually high number of patients with COVID-19 who presented during 8 days with strokes due to ICT within the proximal internal carotid artery lumen. We present the clinical presentation, laboratory studies, and imaging findings of 6 such patients.
Case Series
Institutional review board approval with waived consent was obtained for this Health Insurance Portability and Accountability Act–compliant retrospective Clinical Report. The case series consists of patients with COVID-19 presenting with acute cerebral infarction with varying degrees of intraluminal clot within the internal carotid artery. Patients were selected from those admitted to a large comprehensive stroke center. COVID-19 was diagnosed via detection of SARS-CoV-2 with real-time reverse transcriptase polymerase chain reaction assay performed on specimens obtained via nasopharyngeal or nasal swabs. One patient with real-time reverse transcriptase polymerase chain reaction negative for SARS-CoV-2 but with high suspicion for COVID-19 was also included. That patient had a clinical presentation and laboratory findings typical of COVID-19, as well as chest CT findings of bilateral subpleural consolidative opacities and a large region of ground-glass opacity, which fit the typical appearance of COVID-19 pneumonia according to the Radiological Society of North America Expert Consensus Statement.13 Laboratory values reported include peak levels during the hospitalization course, as well as peak levels within the 24 hours before or after initial identification of ICT on imaging. This is a descriptive study reporting the imaging and clinical characteristics of this series of patients.
Six patients were included (5 men, 1 woman) with a mean age of 65.8 years (range, 55–78 years) (Table). All patients had a history of vascular risk factors, including diabetes (83%), hyperlipidemia (100%), and smoking (17%). Four patients presented with large infarcts with initial NIHSS scores ranging from 24 to 30. Four patients (67%) presented with tandem occlusions identified on CTA. One patient received IV alteplase, and 2 patients underwent mechanical thrombectomy with TICI reperfusion scores ranging from TICI 2a to TICI 2b. With regard to COVID-19 status, 4 patients (67%) had previous symptoms consistent with COVID-19 ranging from 4 to 14 days before the onset of infarction. Two patients (33%) presented with cerebral infarction and later developed respiratory symptoms. One patient tested negative for COVID-19 via nasal swab but was treated as positive for COVID-19 on the basis of clinical, radiographic, and laboratory findings.
Patient demographics and stroke clinical details
During their hospitalization course (On-line Table), all patients (100%) had elevated D-dimer and C-reactive protein levels, 5 (83%) had elevated lactate dehydrogenase and ferritin levels and reduced absolute lymphocyte counts, and 2 (33%) had elevated troponin levels. In addition, of the 4 patients with IL-6 levels tested during their hospitalization, 3 (75%) had elevated levels. Within the 24 hours before or after ICT detection on imaging, of those tested, levels of D-dimer, C-reactive protein, and lactate dehydrogenase were elevated in 100% (5/5 patients tested), absolute lymphocyte counts were reduced in 67% (4/6), ferritin was elevated in 60% (3/5), fibrinogen was elevated in 50% (2/4), and troponin was elevated in 40% (2/5). In addition, of the 3 patients who underwent IL-6 testing during that timeframe, 100% (3/3) had elevated levels. Four patients were significantly debilitated on discharge with mRS scores of 4–5, and 2 patients were discharged with mild deficits (mRS, 1–2).
Patient 1.
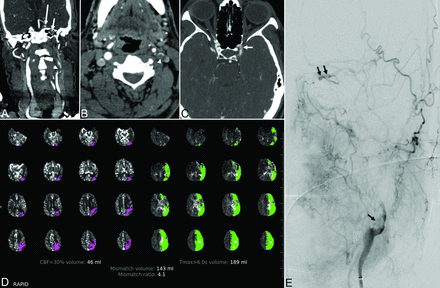
A 55-year-old man with a history of prior acute ischemic stroke and stenosis of the paraclinoid left ICA presented 24 hours after last known well with altered mental status, right hemiplegia, and global aphasia with an initial NIHSS score of 24. Noncontrast CT demonstrated early ischemic changes involving the left thalamus and putamen. CTA showed near-occlusion of the left ICA at the bifurcation due to a large intraluminal clot with complete occlusion more distally and reconstitution at the supraclinoid segment (Fig 1). CT perfusion demonstrated ischemic penumbra and core infarct volumes of 189 and 46 mL, respectively. The patient underwent mechanical thrombectomy of the carotid bifurcation and supraclinoid ICA with removal of a large clot and a final TICI 2a reperfusion grade.
Patient 1. A 55-year-old man with COVID-19 and an NIHSS score of 24. A and B, Coronal reformatted and axial images from CT angiography of the head and neck demonstrate an irregular plaque at the left internal carotid artery bifurcation (arrowhead) and nonopacification of the high cervical and petrous (short arrow) left internal carotid artery from tandem occlusion, with reconstitution of the supraclinoid segment (long arrow). C, CT angiography 3 years prior shows moderate focal stenosis of the left paraclinoid ICA (white arrow). D, CT perfusion map shows a large volume of hypoperfusion (time-to-maximum > 6 seconds) involving the left anterior and middle cerebral artery territory and a considerably smaller volume of presumed core infarct (CBF of <30%) in the left parietal region. E, Digital subtraction angiography of the left internal carotid artery demonstrates an eccentric filling defect of the left carotid bifurcation (black arrow) and left ICA occlusion, with recanalization at the level of the left ophthalmic artery from external-to-internal carotid collaterals (double black arrow).
Patient 2.
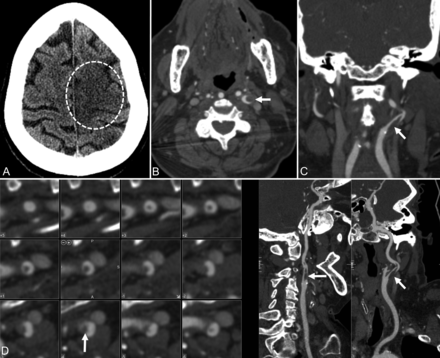
A 78-year-old woman presented with a 1-week history of fatigue, anorexia, headache, and respiratory distress. Following admission with bilateral air space opacities suggestive of multifocal pneumonia, the patient's condition stabilized and she was weaned off supplemental oxygen. The next morning the patient was found to be mute and hemiplegic on the right with an initial NIHSS score of 25. Noncontrast CT demonstrated evolving ischemic changes in the left caudate nucleus, putamen, and left frontal and parietal cortices (Fig 2). CTA demonstrated an irregular plaque at the left ICA bifurcation and intraluminal clot extending superiorly. The patient did not receive thrombolytic therapy because she was outside the treatment window of 4.5 hours. She did not undergo mechanical thrombectomy because no LVO was present on CTA.
Patient 2. A 78-year-old woman with COVID-19 and an NIHSS score of 25. A, CT of the head without contrast demonstrates an evolving ischemic infarct in the left frontoparietal paracentral cortex (dotted circle) with a smaller infarct posteriorly in the left parietal cortex. B–D, Axial, coronal, and curved reformatted images from CT angiography of the head and neck demonstrate an irregular plaque at the left internal carotid artery bifurcation and an intraluminal filling defect (arrow) extending superiorly in the left internal carotid artery, corresponding to ruptured plaque with clot formation.
Patient 3.
A 62-year-old man with a history of diabetes and hypertension presented with sudden-onset left-sided weakness. On arrival, the patient was noted to be hypoxic requiring oxygen supplementation, and his physical examination was notable for left hemiplegia and dysarthria with an initial NIHSS score of 25. Noncontrast CT demonstrated an acute infarct involving the right frontal and temporal lobes. CTA demonstrated complete occlusion of the right ICA from its origin with reconstitution at the paraclinoid segment. Distally, there was occlusion of the mid-to-distal M1 segment of the right MCA. The patient did not receive thrombolytic therapy because he was discovered 18 hours after symptom onset, and mechanical thrombectomy was not performed due to a large established infarct on noncontrast CT.
Patient 4.
A 74-year-old man with a medical history of hypertension, chronic kidney disease, and type 2 diabetes presented with altered mental status and hypoxia. On examination, the patient had a mild right facial droop and hemiparesis, with an initial NIHSS score of 5. Noncontrast CT demonstrated no acute abnormality. CT angiography showed thrombus in the right carotid bulb extending into the proximal right internal carotid artery. The patient underwent emergent carotid endarterectomy.
Patient 5.
A 59-year-old man presented with sudden-onset speech changes. The physical examination was notable for slurred speech and an initial NIHSS of 1. Noncontrast CT demonstrated hyperdensity involving the M2 segment of the right MCA. CTA confirmed occlusion of an M2 segment branch of the right MCA in addition to a noncalcified low-density filling defect along the wall of the right ICA immediately distal to the bifurcation, consistent with a ruptured atherosclerotic plaque with adherent thrombus. The patient was treated with IV alteplase, resulting in improvement of the neurologic deficits. MR imaging demonstrated an acute infarct involving the right frontal, temporal, and parietal lobes.
Patient 6.
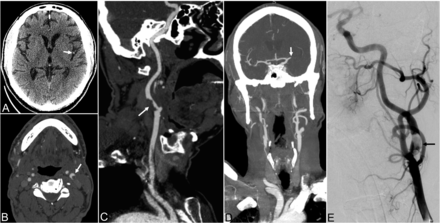
A 67-year-old man with a medical history of hyperlipidemia presented with fever and sudden loss of consciousness. On examination, the patient had left-gaze deviation, dense left hemiplegia, and aphasia with an initial NIHSS of 30. Noncontrast CT revealed a hyperdense left MCA, but otherwise no acute process (Fig 3). CTA showed near-occlusive ruptured plaque and thrombus in the left ICA origin, along with complete occlusion of the M1 segment of the left MCA. CT perfusion demonstrated ischemic penumbra and core infarct volumes of 159 and 36 mL, respectively. The patient underwent mechanical thrombectomy of the carotid bifurcation and supraclinoid ICA with a final TICI 2b reperfusion grade after removal of 2 separate clots. The patient's physical examination findings following intervention were notable for improvement in language but continued right hemiplegia and dysphagia.
Patient 6. A 67-year-old man presenting with fever and sudden loss of consciousness. A, Axial noncontrast CT of the head demonstrates a hyperdense left MCA branch (arrow) without evidence of acute infarct. B and C, Axial and curved reformatted images from CT angiography of the head and neck demonstrate an intraluminal clot projecting into the left internal carotid artery at the bifurcation (arrows). D, Coronal MIP from CT angiography of the head and neck demonstrates occlusion of the M1 segment of the left MCA. E, Digital subtraction angiography of the left internal carotid artery demonstrates an eccentric filling defect of the left carotid bifurcation (black arrow).
DISCUSSION
These cases demonstrate 6 patients with COVID-19 (5 confirmed, 1 presumed) presenting with acute ischemic infarction. Each patient had evidence of ICT within the proximal internal carotid artery, most likely secondary to plaque rupture followed by thrombus formation and subsequent artery-to-artery embolism. There is evidence that inflammation promotes the development of atherosclerosis and is also associated with plaque rupture.14 The Canakinumab Anti-Inflammatory Thrombosis Outcome Study noted that patients who received an IL-1 inhibitor had significantly decreased levels of IL-6 and reduced rates of myocardial and cerebral infarction.15
We suspect that in our cases, the proinflammatory state caused by COVID-19 infection contributed to plaque instability and rupture, with subsequent thrombosis promoted by the thrombogenic environment also caused by the ongoing COVID-19 infection. There is evidence suggesting that cytokine storms play a role in cases of severe COVID-19; and for some patients, this inflammatory state, coupled with the thrombogenic environment, may play an important role in clot formation and subsequent infarction. All patients in this series had a history of vascular risk factors, including diabetes, hyperlipidemia, and smoking, which may have increased their susceptibility to plaque rupture and thrombosis.
Notably, all 6 patients presented with no-or-mild COVID-19 symptoms, and 5 of the 6 patients (83%) presented with stroke symptoms as the chief symptoms. This suggests that even mild cases of COVID-19 can result in thrombogenicity, inflammation, and ultimately plaque rupture and thrombosis. In previous studies examining complications related to coronavirus-related diseases, most complications were seen in critically ill patients.16,17 However, the fact that most of our patients had milder COVID-19 symptoms suggests that a contributing factor may be a direct effect of the SARS-CoV-2 infection rather than systemic inflammation alone or a postinfectious prothrombotic state.
Viral infections are known to have the potential for causing vascular disease, including through atherogenesis, endothelial activation leading to altered coagulation and fibrinolytic systems, as well as direct endothelial cell invasion.18 In addition, recent studies have found evidence of direct infection of the endothelium by SARS-CoV-2 with diffuse endothelial inflammation, further supporting the role of the virus in these cases.19 The fact that all patients presented with involvement at the carotid bifurcation may be due to its pre-existing propensity for atherosclerosis and turbulent flow; the latter can damage the endothelium and lead to platelet aggregation.
The limitations of this study include the inability to prove causality between COVID-19 infection and ICT due to the retrospective nature of the study and the small number of patients. Additionally, there was neither consistent assessment of the same systemic inflammatory markers in all patients nor pathologic confirmation of the embolic material or carotid plaque. Finally, baseline and follow-up arterial wall imaging beyond CTA was not performed.
Our findings suggest that there is an increased risk of LVO due to ICT in patients with COVID-19 infection who have typical vascular risk factors. Additionally, our patients most often presented with symptoms of cerebral ischemia rather than typical COVID-19 symptoms. Furthermore, cerebral ischemia due to ICT was not limited to patients with severe COVID-19 symptoms. The possible coincidence of cerebral ischemia due to ICT and COVID-19 infection is an essential consideration, particularly in the evaluation of encephalopathy, which is common with viral illnesses. This recognition is important to ensure that an appropriate diagnostic work-up is performed, including carotid imaging, as well as the timely treatment of acute cerebral ischemia and initiation of antithrombotic therapy for stroke prevention.
Footnotes
Ali Y. Mohamud and Brent Griffith contributed equally to this work.
Disclosures: Ali Y. Mohamud—UNRELATED: Employment: Henry Ford Health System, Comments: current postgraduate year 2 neurology resident. Mohammed Rehman—UNRELATED: Payment for Lectures Including Service on Speakers Bureaus: Portola Pharmaceuticals, American Academy of Neurology. Daniel Miller—UNRELATED: Payment for Lectures Including Service on Speakers Bureaus: Boston Scientific, BioTelemetry Inc, Comments: 1 engagement each; both unrelated to this topic. Alex Chebl—UNRELATED: Payment for Lectures Including Service on Speakers Bureaus: Medtronic, Comments: speaker 23 months ago.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received May 9, 2020.
- Accepted after revision June 1, 2020.
- © 2020 by American Journal of Neuroradiology