Summary
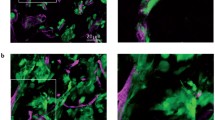
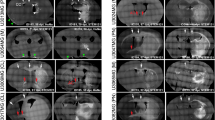
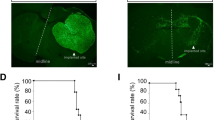
We present morphological data of the early stage of tumor invasion in the central nervous system. C6 rat glioma cells were injected into the caudate-putamen of rat brain using glass micropipettes to minimize traumatic reactions. Four days after the inoculation, we examined the tumor-brain interface using light and electron microscopy. Ultrastructurally the tumor processes were attached to the perivascular basement membrane instead of the astroglial end-feet. At the tumor periphery, the vessel walls were in contact with both tumor processes and astroglial end-feet. Astrocytes withdrew their processes from the vascular walls and changed into a reactive phenotype, while the neuronal cells remained virtually intact, even when surrounded by tumor cells. Immunohistochemical study using C6 cells labeled with bromodeoxyuridine showed migration of the cells toward the perviascular space that was distant from the site of injection. These observations represent the earliest morphologically detectable changes of the tumor-brain interface, and suggest that the C6 cells possess the characteristics of high affinity to the endothelial basement membrane and invade along the preexisting blood vessels with brain parenchymal infiltration.
Similar content being viewed by others
References
Auer RN, Del Maestro RF, Anderson R (1981) A simple and reproducible experimental in vivo glioma model. Can J Neurol Sci 8: 325–331
Barde YA, Lindsay RM, Monard D, Thoenen H (1978) New factor released by cultured glioma cells supporting survival and growth of sensory neurons. Nature 274: 818
Barker M, Hoshino T, Gurcay O, Wilson CB, Nielson SL, Downie R, Eliason J (1973) Development of an animal brain tumor model and its response to therapy with 1,3-bis(2-chloroethyl)-1-nitrosourea. Cancer Res 33: 976–986
Benda P, Lightbody J, Sato G, Levine L, Sweet W (1968) Differentiated rat glial cell strain in tissue culture. Science 161: 370
Bernstein JJ, Getz R, Jefferson M, Kelemen M (1985) Astrocytes secrete basal lamina after hemisection of rat spinal cord. Brain Res 327: 135–141
Bissell MG, Rubinstein LJ, Bignami A, Herman MM (1974) Characteristics of the rat C-6 glioma maintained in organ culture systems. Production of glial fibrillary acidic protein in the absence of gliofibrillogenesis. Brain Res 82: 77–89
Carter WG, Wayner EA, Bouchard TS, Kaur P (1990) The role of integrins α2β1, and α3β1 in cell-cell and cell-substrate adhesion of human epithelial cells. J Cell Biol 110: 1387–1404
Cerame MA, Guthikonda M, Kohli CM (1985) Extraneural metastasis in gliosarcoma: a case report and review of the literature. Neurosurgery 17: 413–418
Couldwell WT, de Tribolet N, Antel JP, Gauthier T, Kuppner MC (1992) Adhesion molecules and malignant gliomas: implications for tumorigenesis. J Neurosurg 76: 782–791
Eng LR, Reier PJ, Houle JD (1987) Astrocyte activation and fibrous gliosis: glial fibrillary acidic protein immunostaining of astrocyte following intraspinal cord grafting of fetal CNS tisue. Prog Brain Res 71: 439–445
Farrell CL, Stewart PA, Del Maestro RF (1987) A new glioma model in rat: the C6 spheroid implantation technique permeabiligy and vascular characterization. J Neurooncol 4: 403–415
Hürter T, Mennel HD (1981) Experimental brain tumors and edema in rats. I. Histology and cytology of tumors. Acta Neuropathol (Berl) 55: 105–111
Jänisch W, Schreiber D (1977) Methods of induction of experimental CNS tumors. In: Binger DD, Swenberg JA (eds), Experimental tumors of the central nervous system, 1st English edn. The UpJohn Co, Kalamazoo, pp 16–41
Jones PA, De Clerck YA (1982) Extracellular matrix destruction by invasive tumor cells. Cancer Metastasis Rev 1: 289–297
Kleihues P, Lantos PL, Magee PN (1976) Chemical carcinogenesis in the nervous system. Int Rev Exp Pathol 15: 153–232
Klein G, Klien E (1985) Evolution of tumours and the impact of molecular oncology. Nature 315: 190–195
Lantos PL, Pilkington GJ (1979) The development of experimental brain tumours. A sequential light and electron microscope study of the subependymal plate. I. Early lesions (abnormal cell clusters). Acta Neuropathol (Berl) 45: 167–175
Liepkalns VA, Icard-Liepkalns C, Sommer AM, Quigley JP (1982) Properties of cloned human glioblastima cells. J Neurol Sci 57: 257–264
Lindsay RM (1986) Reactive gliosis. In: Federoff S, Vernadakis A (eds) Astrocytes, vol. 3. Academic Press, New York, pp 231–262
Manthorpe MM, Rudge JS, Varon S (1986) Astroglial cell contributions to neuronal survival and neuritic growth. In: Federoff S, Vernadakis A (eds) Astrocytes, vol 3. Academic Press, New York, pp 315–376
Mareel MM, Bracke ME, Boghaert ER (1986) Tumor invasion and metastasis: therapeutic implications? Radiother Oncol 6: 135–142
Mørk S, Laerum OD, de Ridder L (1984) Invasiveness of tumours of the central nervous system. In: Mareel MM, Calman K (eds) Tumor invasion. Oxford University Press, New York, pp 79–125
Murray KJ, Chou SN, Douglas SD (1976) Topographical anatomy of human cerebral and cerebellar astrocytomas in vitro. Surg Neurol 6: 337–340
Nicolson GL (1982) Cancer metastasis: organ colonization and the cell-surface properties of malignant cells. Biochim Biophys Acta 695: 113–176
Paganetti PA, Caroni P, Schwab ME (1988) Glioblastoma infiltration into central nervous system tissue in vitro: involvement of a metalloprotease. J Cell Biol 107: 2281–2291
Parker KK, Norenberg MD, Vernadakis A (1980) “Transdifferentiation” of C6 glial cells in culture. Science 208: 179–181
Pauli BU, Schwartz DE, Thonar EJ-M, Kuettner KE (1983) Tumor invasion and host extracellular matrix. Cancer Metastasis Rev 2: 129–152
Reier PJ (1986) Gliosis following CNS injury: the anatomy of astrocytic scars and their influences on axonal elongation. In: Federoff S, Vernadakis A (eds) Astrocytes, vol 3. Academic Press, New York, pp 263–324
Rutka JT (1986) Effects of extracellular matrix proteins on the growth and differentiation of an anaplastic glioma cell line. Can J Neurol Sci 13: 301–306
Rutka JT, Apodaka G, Stern R, Rosenblum M (1988) The extracellular matrix of the central and peripheral nervous system: structure and function. J Neurosurg 69: 155–170
Sasaki H, Sato F, Mannen H (1989) Morphological analysis of single astrocytes of the adult cat central nervous system visualized by HRP microinjection. Brain Res 501: 339–354
Schwartz MS, Morris J, Sarid J (1991) Overexpression of oncogene products can cause tumor progression without parenchymal infiltration in the rat brain. Cancer Res 51: 3595–3601
Shabad LM (1973) Precancerous morphologic lesions. J Natl Cancer Inst 50: 1421–1428
Spence AM, Coates PW (1978) Scanning electron microscopy of cloned astrocytic lines derived from ethylnitrosoureainduced rat gliomas. Virchows Arch [B] 28: 77–85
Stewart PA, Hayakawa K, Hayakawa E, Farrell CL, Del Maestro RF (1985) A quantitative study of blood-brain barrier permeability ultrastructure in a new rat glioma model. Acta Neuropathol (Berl) 67: 96–102
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nagano, N., Sasaki, H., Aoyagi, M. et al. Invasion of experimental rat brain tumor: early morphological changes following microinjection of C6 glioma cells. Acta Neuropathol 86, 117–125 (1993). https://doi.org/10.1007/BF00334878
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00334878