Summary
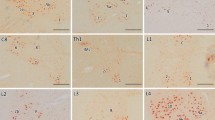
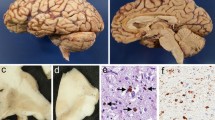
We examined patterns of neuronal degeneration in the motor cortex of amyotrophic lateral selerosis (ALS) patients using traditional cell stains and several histochemical markers including neurofilament, parvalbumin, NADPH-diaphorase, ubiquitin, Alz-50 and tau. Three grades of ALS (mild, moderate, severe) were defined based on the extent of Betz cell depletion. Non-phosphorylated neurofilament immunoreactive cortical pyramidal neurons and non-pyramidal parvalbumin local circuit neurons were significantly depleted in all grades of ALS. In contrast, NADPH-diaphorase neurons and Alz-50-positive neurons were quantitatively preserved despite reduced NADPH-diaphorase cellular staining and dendritic pruning. The density of ubiquitin-positive structures in the middle and deep layers of the motor cortex was increased in all cases. Axonal tau immunoreactivity was not altered. These histochemical results suggest that cortical degeneration in ALS is distinctive from other neurodegenerative diseases affecting cerebral cortex. Unlike Huntington's disease, both pyramidal and local cortical neurons are affected in ALS; unlike Alzheimer's disease, alteration of the neuronal cytoskeleton is not prominent. The unique pattern of neuronal degeneration found in ALS motor cortex is consistent with non-N-methyl-Dxxx-aspartate glutamate receptor-mediated cytotoxicity.
Similar content being viewed by others
References
Arai H, Emson PC, Mountjoy CQ, Carassco LH, Heinzmann CW (1987) Loss of parvalbumin immunoreactive neurons from cortex in Alzheimer-type dementia. Brain Res 418:164–169
Arai H, Lee VM-Y, Otvos L, Greenberg BD, Lowery DE, Sharma SK, Schmidt ML, Trojanowski JQ (1990) Defined neurofilament, tau, and β-amyloid precursor protein epitopes distinguish Alzheimer from non-Alzheimer senile plaques. Proc Natl Acad Sci USA 87:2249–2253
Beal MF, Swartz KJ, Finn SF, Mazurek MF, Kowall NW (1991) Neurochemical characterization of excitoxin lesions in the cerebral cortex. J Neurosci 11:147–158
Berchtold MW, Celio MR, Heinzmann CW (1985) Parvalbumin in human brain. J Neurochem 45:235–239
Brownell B, Oppenheimer DR, Hughes JT (1970) The central nervous system in motor neurone disease. J Neurol Neurosurg Psychiatry 33:338–357
Campbell MJ, Morrison JH (1989) Monoclonal antibody to neurofilament protein (SMI-32) labels a subpopulation of pyramidal neurons in the human and monkey neocortex. J Comp Neurol 282:191–205
Campbell SK, Switzer RC, Martin TL (1987) Alzheimer's plaques and tangles: a controlled and enhanced silver-staining method. Soc Neurosci Abstr 13:678
Carpenter S (1968) Proximal axonal enlargement in motor neuron disease. Neurology 18:841–851
Chase RA, Pearson S, Nunn PB, Lantos PL (1985) Comparative toxicities of alpha-and beta-N-oxalyl-Lxxx-alpha, beta-diaminopropionic acids to rat spinal cord. Neurosci Letter 55:89–94
Chou SM (1991) Neuropathology of upper motor neurons in amyotrophic lateral slcerosis. Proc XI Intl Cong Neuropathol, pp 595–598
Cudkowicz M, Kowall NW (1990) Degeneration of pyramidal projection neurons in Huntington's disease cortex. Ann Neurol 27:200–204
Cudkowicz M, Kowall NW (1990) Parvalbumin-immunoreactive neurons are resistant to degeneration in Huntington's disease cerebral cortex. J Neuropathol Exp Neurol 49:345
Dalakas MC, Hatazawa J, Brooks A, Chiro GD (1987) Lowered cerebral glucose utilization in amyotrophic lateral sclerosis. Ann Neurol 22:580–586
Delisle MB, Carpenter S (1984) Neurofibrillary axonal swellings and amyotrophic lateral sclerosis. J Neurosci 63:241–250
Dickson DW, Wertkin A, Kress Y, Ksiezak-Reding H, Yen S-H (1990) Ubiquitin immunoreactive structures in normal human brains. Distribution and developmental aspects. Lab Invest 63:87–99
Dowson VL (1991) Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci. USA 88:6368–6371
Ferrante RJ, Kowall NW, Beal MF, Richardson JEP Bird ED, Martin JB (1985) Selective sparing of a class of striatal neurons in Huntington's disease. Science 230:561–563
Ferrante RJ, Kowall NW, Richardson EP (1991) Proliferative and degenerative changes in striatal spiny neurons in Huntington's disease: a combined study using the section-Golgi method and calbinidin D28K immunocytochemistry. J Neurosci 11:3877–3887
Ferrer I, Soriano E, Tunon T, Fonseca M, Guionnet N (1991) Parvalbumin immunoreactive neurons in normal human temporal neocortex and in patients with Alzheimer's disease. J Neurol Sci 106:135–141
Gold BG, Griffen JW, Price DL (1985) Slow axonal transport in acrylamide neuropathy: different abnormalities produced by a single dose and continuous administration. J Neurosci 5:1755–1768
Hall GF, Cohen MJ (1988) The pattern of dendritic sprouting and retraction induced by axotomy of Lamprey central neurons. J Neurosci 8:3584–3597
Hatazawa J, Brooks RA, Dalakas MC, Mansi L, Di Chiro G (1988) Cortical motor-sensory hypometabolism in amyotrophic lateral sclerosis: a PET study. J Comput Assist Tomogr 12:630–636
Heinzmann CW (1984) Parvalbumin, an intracellular calciumbinding protein: distribution, properties and possible roles in mammalian cells. Experientia 40:910–921
Hof PR, Morrison JH (1990) Quantitative analysis of a vulnerable subset of a pyramidal; neurons in Alzheimer's disease. II. Primary and secondary visual cortex. J Comp Neurol 301:55–64
Hof PR, Cox K, Morrison JH (1990) Quantitative analysis of a vulnerable subset of pyramidal neurons in Alzheimer's disease. 1. Superior frontal and inferior temporal cortex. J Comp Neurol 301P44–54
Hugon J, Vallat JM, Spencer PS, Leboutet MJ, Barthe D (1989) Kainic acid induces early and delayed degenerative neuronal changes in rats spinal cord. Neurosci Letter 104:258–262
Ihara Y (1988) Massive somatodendritic sprouting of cortical neurons in Alzheimer's disease. Brain Res 459:138–144
Koh J-Y, Choi DW (1988) Vulnerability of cultured cortical neurons to damage by excitotoxins: differential susceptibility of neurons containing NADPH-diaphorase. J Neurosci 8:2153–2163
Kowall NW, Beal MF (1988) Cortical somatostatin, neuropeptide Y and NADPH-diaphorase neurons: normal anatomy and alterations in Alzheimer's disease. Ann Neurol 23:105–114
Kowall NW, Kosik KS (1987) Axonal disruption and aberrant localization of tau protein characterize the neuropil pathology of Alzheimer's disease. Ann Neurol 22:639–643
Kowall NW, Ferrante RJ, Martin JB (1987) Patterns of cell loss in Huntington's disease. Trends Neurosci 10:24–29
Kuncl RW, Crawford TO, Rothstein JD, Drachman DB (1992) Motor neuron diseases. In: Asbury AK, McKahnn GM, McDonald WI (eds) Diseases of the nervous system. Clinical neurobiology, vol 2. WB Saunders, Philadelphia, pp 1179–1208
Manetto V, Sternberger NH, Perry G,Sternberger LA, Gambetti P (1988) Phosphorylation of neurofilaments is altered in amyotrophic lateral sclerosis. J Neuropathol Exp Neurol 47:642–653
McKee AC, Kosik KS, Kowall NW (1991) Neuritic pathology and dementia in Alzheimer's disease. Ann Neurol 30:156–165
McKee AC, Kowall NW, Kosik KS (1989) Microtubular reorganization and dendritic growth response in Alzheimer's disease. Ann Neurol 26:652–659
Murayama S, Bouldin TW, Suzuki K (1992) Immunocytochemical and ultrastructural studies of upper motor neurons in amyotrophic lateral sclerosis. Acta Neuropathol 83:518–524
Nag S, Riopelle RJ (1990) Spinal neuronal pathology associated with continuous intrathecal infusion of N-methyl-Dxxx-aspartate in the rat. Acta Neuropathol 81:7–13
Neary D, Snowden JS, Mann DMA, Northen B, Gouilding PJ, MacDermott N (1990) Frontal lobe dementia and motor neuron disease. J Neurol Neurosurg Psychiatry 53:23–32
Nihei K, Kowall NW (1992) Neurofilament and neural cell adhesion molecule immunocytochemistry of Huntington's disease striatum. Ann Neurol 31:59–63
Nihei K, Kowall NW, McKee AC (1992) Degeneration of non pyramidal local circuit neurons in the motor cortex of amyotrophic lateral sclerosis patients (abstract). J Neuropathol Exp Neurol 51:322
Ohshima T, Endo T, Onaya T (1991) Distribution of parvalbumin immunoreactivity in the human brain. J Neurol 238:320–322
Olney JW (1978) Neurotoxicity of excitatory amino acids. In: McGeer EG, Olney JW, McGeer PL (eds) Kainic acid as a tool in neurobiology. Raven Press, New York, pp 95–121
Oppenheimer DR, Esiri MM (1992) Diseases of the basal ganglia, cerebellum and motor neurons. In: Adams JH, Duchen LW (eds) Greenfield's neuropathology. Oxford University Press, New York, pp 1021–1029
Pappolla MA, Omar R, Saran B (1989) The “normal” brain: “Abnormal” ubiquitinated deposits highlight an age-related protein change. Am J Pathol 135:585–591
Perry T, Hansen S, Jones K (1987) Brain glutamate deficiency in amyotrophic lateral sclerosis. Neurology 37:1845–1848
Pisharodi M, Nauta JHW (1985) An animal model for neuron-specific spinal cord lesions by the microinjection of N-methylaspartate, kainic acid, and quisqualic acid. Appl Neurophysiol 48:226–233
Plaitakis A, Caroscio JT (1987) Abnormal glutamate metabolism in amyotrophic lateral slcerosis. Ann Neurol 22:575–579
Rothstein JD, Tsai G, Kuncl RW, Clawson L, Gornblath DR, Drachman dB, Pestronk A, Stauch BL, Coyle JT (1990) Abnormal excitatory amino acid metabolism in ALS. Ann Neurol 28:18–25
Rothstein JD, Martin LJ, Kuncl RW (1992) Decreased glutamate transport by brain and spinal cord in amyotrophic lateral sclerosis. N Engl J Med 326:1464–1468
Satoh J, Tabira T, Sano M, Nakayama H, Tateishi J (1991) Parvalbumin-immunoreactive neurons in the human central nervous system are decreased in Alzheimer's disease. Acta Neuropathol 81:388–395
Scherer-Singer U, Vincent SR, Kimura H, McGeer EG (1983) Demonstration of a unique population of neurons with NADPH-diaphorase histochemistry. J Neurosci Methods 9:229–234
Schmidt ML, Carden MJ, Lee VM-Y, Trojanowski JQ (1987) Phosphate dependent and independent neurofilament epitopes in the axonal swellings of patients with motor neuron disease and control. Lab Invest 56:282–294
Sholl DA (1953) Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat 83:387–407
Stewart GR, Olnery JW, Pathikonda M, Snider WD (1991) Excitotoxicity in the embryonic chick spinal cord. Ann Neurol 30:758–766
Storey E, Kowall NW, Finn SF, Mazurek MF, Beal MF (1992) The cortical lesion of Huntington's disease: further neurochemical characterization, and reproduction of some of the histologic and neurochemical features by N-methyl-Dxxx-aspartate lesions of rat cortex. Ann Neurol 32:526–534
Tandan R, Robinson SH, Munzer JS, Bradley WG (1987) Deficient DNA rapair in amyotrophic lateral sclerosis cells. J neurol Sci 79:189–203
Tandan R (1991) Disorders of the upper and lower motor neurons. In: Bradley WG, Daroff RB, Fenichel GM, Marsden CD (eds) Neurology in clinical practice. The neurological disorders. vol 2. Butterworth-Heinemann, Stoneham, pp 1694–1707
Thomas E, Pearse AGE (1964) The solitary active cells: histochemical demonstration of damage resistant nerve cells with a TPN-diaphorase reaction. Acta Neuropathol (Berl) 3:238–249
Troncoso JC, Hoffman PN, Griffen JW, Hess-Kozlow KM, Price DL (1985) Aluminium intoxication: a disorder of neurofilament transport in motor neurons. Brain Res 342:172–175
Weiss JH, Koh J-Y, Baimbridge KG, Choi DW (1990) Cortical neurons containing somatostatin-or parvalbumin-like immunoreactivity are atypically vulnerable to excitotoxic injury in vitro. Neurology 40:1288–1292
Wightman G, Anderson VER, Martin J, Swash M, Anderson BH, Neary D, Mann D, Luthert P, Leigh PN (1992) Hippocampal and neocortical ubiquitin-immunoreactive inclusions in amyotrophic lateral sclerosis with dementia. Neurosci Lett 139:269–274
Wolozin BL, Pruchnicki A, Dickson PD (1986) A neuronal antigen in the brains of Alzheimer patients. Science 232:648–650
Wolozin BL, Scicutella A, Davies P (1988) Expression of a developmentally regulated antigen in Down syndrome and Alzheimer's disease. Proc Natl Acad Sci USA 85:6202–6206
Wong J, Hutchison SB, Liem RKH (1984) An isoelectric variant of the 150,000-dalton neurofilament polypeptide. Evidence that phosphorylation state affects its association with the filament. J Biol Chem 259:10867–10874
Author information
Authors and Affiliations
Additional information
Supported in part by a Muscular Dystrophy Association Research Development grant
Rights and permissions
About this article
Cite this article
Nihei, K., McKee, A.C. & Kowall, N.W. Patterns of neuronal degeneration in the motor cortex of amyotrophic lateral sclerosis patients. Acta Neuropathol 86, 55–64 (1993). https://doi.org/10.1007/BF00454899
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00454899