Abstract
The cerebral cortex reorganizes in response to central or peripheral lesions. Although basal ganglia and cerebellum are key components of the network dedicated to movement control, their role in motor reorganization remains elusive. We therefore tested if slowly progressive neurodegenerative motor disease alters the subcortical functional anatomy of the basal ganglia-thalamo-cerebellar circuitry. Ten patients with amyotrophic lateral sclerosis (ALS) and ten healthy controls underwent functional magnetic resonance imaging (fMRI), while executing a simple finger flexion task. Cued by an acoustic trigger, they squeezed a handgrip force transducer with their right hand at 10% of their maximum voluntary contraction force. Movement frequency, amplitude, and force were controlled. Statistical parametric mapping of task-related BOLD-response revealed increased activation in ALS patients as compared to healthy controls. The main activation increases were found in the supplementary motor area, basal ganglia, brainstem, and cerebellum. These findings suggest that degeneration of cortical and spinal motor neurons in ALS leads to a recruitment of subcortical motor structures. These subcortical activation patterns strongly resemble functional activation in motor learning and might therefore represent adaptations of cortico-subcortical motor loops as a—albeit finally ineffective—mechanism to compensate for the ongoing loss of motor neurons in ALS.



Similar content being viewed by others
References
Alexander GE, Crutcher MD (1990) Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci 13:266–271
Allison JD, Meador KJ, Loring DW, Figueroa RE, Wright JC (2000) Functional MRI cerebral activation and deactivation during finger movement. Neurology 54:135–142
Blakemore SJ, Frith CD, Wolpert DM (2001) The cerebellum is involved in predicting the sensory consequences of action. Neuroreport 12:1879–1884
Brooks BR (1994) El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial “Clinical limits of amyotrophic lateral sclerosis” workshop contributors. J Neurol Sci 124(Suppl):96–107
Brooks BR, Bushara K, Khan A, Hershberger J, Wheat JO, Belden D, Henningsen H (2000) Functional magnetic resonance imaging (fMRI) clinical studies in ALS–paradigms, problems and promises. Amyotroph Lateral Scler Other Motor Neuron Disord 1(Suppl 2):S23–32
Bruehlmeier M, Dietz V, Leenders KL, Roelcke U, Missimer J, Curt A (1998) How does the human brain deal with a spinal cord injury? Eur J Neurosci 10:3918–3922
Byrnes ML, Thickbroom GW, Phillips BA, Mastaglia FL (2001) Long-term changes in motor cortical organisation after recovery from subcortical stroke. Brain Res 889(1–2):278–287
Cao Y, D’Olhaberriague L, Vikingstad EM, Levine SR, Welch KM (1998) Pilot study of functional MRI to assess cerebral activation of motor function after poststroke hemiparesis. Stroke 29:112–122
Carpentier AC, Constable RT, Schlosser MJ, de Lotbiniere A, Piepmeier JM, Spencer DD, Awad IA (2001) Patterns of functional magnetic resonance imaging activation in association with structural lesions in the rolandic region: a classification system. J Neurosurg 94:946–954
Chapman LJ, Chapman JP (1987) The measurement of handedness. Brain Cogn 6:175–183
Chollet F, DiPiero V, Wise RJ, Brooks DJ, Dolan RJ, Frackowiak RS (1991) The functional anatomy of motor recovery after stroke in humans: a study with positron emission tomography. Ann Neurol 29:63–71
Dettmers C, Connelly A, Stephan KM, Turner R, Friston KJ, Frackowiak RS, Gadian DG (1996) Quantitative comparison of functional magnetic resonance imaging with positron emission tomography using a force-related paradigm. Neuroimage 4:201–209
Doyon J, Benali H (2005) Reorganization and plasticity in the adult brain during learning of motor skills. Curr Opin Neurobiol 15:161–167
Doyon J, Penhune V, Ungerleider LG (2003) Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia 41:252–262
Flament D, Ellermann JM, Kim SG, Ugurbil K, Ebner TJ (1996) Functional magnetic resonance imaging of cerebellar activation during the learning of a visuomotor dissociation task. Hum Brain Mapp 4:210–226
Friston KJ, Holmes AP, Price CJ, Buchel C, Worsley KJ (1999a) Multisubject fMRI studies and conjunction analyses. Neuroimage 10:385–396
Friston KJ, Holmes AP, Worsley KJ (1999b) How many subjects constitute a study? Neuroimage 10:1–5
Gandevia SC, McCloskey DI (1977) Sensations of heaviness. Brain 100:345–354
Graybiel AM (2000) The basal ganglia. Curr Biol 10:R509–R511
Hammer RP Jr, Tomiyasu U, Scheibel AB (1979) Degeneration of the human Betz cell due to amyotrophic lateral sclerosis. Exp Neurol 63:336–346
Henningsen H, Knecht S, Deppe M, Bremer J, Mock B, Konrad C, Kolan M, Wheat J, Edgar T, Sorenson JA, Turski P, Brooks BR (1998) Common recruitment pattern of associative motor areas in patients with degeneration of cortical pyramidal cells, as measured by fMRI. Neuroimage 7:S1001
Hudson AJ, Kiernan JA, Munoz DG, Pringle CE, Brown WF, Ebers GC (1993) Clinicopathological features of primary lateral sclerosis are different from amyotrophic lateral sclerosis. Brain Res Bull 30:359–364
Jenkins IH, Brooks DJ, Nixon PD, Frackowiak RS, Passingham RE (1994) Motor sequence learning: a study with positron emission tomography. J Neurosci 14:3775–3790
Jueptner M, Frith CD, Brooks DJ, Frackowiak RS, Passingham RE (1997a) Anatomy of motor learning. II. Subcortical structures and learning by trial and error. J Neurophysiol 77:1325–1337
Jueptner M, Stephan KM, Frith CD, Brooks DJ, Frackowiak RS, Passingham RE (1997b) Anatomy of motor learning. I. Frontal cortex and attention to action. J Neurophysiol 77:1313–1324
Kew JJ, Leigh PN, Playford ED, Passingham RE, Goldstein LH, Frackowiak RS, Brooks DJ (1993) Cortical function in amyotrophic lateral sclerosis. A positron emission tomography study. Brain 116:655–680
Kew JJ, Brooks DJ, Passingham RE, Rothwell JC, Frackowiak RS, Leigh PN (1994) Cortical function in progressive lower motor neuron disorders and amyotrophic lateral sclerosis: a comparative PET study. Neurology 44:1101–1110
Kiernan JA, Hudson AJ (1991) Changes in sizes of cortical and lower motor neurons in amyotrophic lateral sclerosis. Brain 114:843–853
Kilgard MP, Merzenich MM (1998) Cortical map reorganization enabled by nucleus basalis activity. Science 279:1714–1718
Konrad C, Henningsen H, Bremer J, Mock B, Deppe M, Buchinger C, Turski P, Knecht S, Brooks BR (2002) Pattern of cortical reorganization in amyotrophic lateral sclerosis: a functional magnetic resonance imaging study. Exp Brain Res 143:51–56
Konrad C, Henningsen H, Jansen A, Knecht S (2005) Comparing brain activation across groups with different motor abilities. J Neurol: DOI: 10.1007/s00415-005-0973-y
Krings T, Topper R, Willmes K, Reinges MH, Gilsbach JM, Thron A (2002) Activation in primary and secondary motor areas in patients with CNS neoplasms and weakness. Neurology 58:381–390
Lawyer T, Netsky MG (1953) Amyotrophic lateral sclerosis. A clinicoanatomical study of fifty-three cases. AMA Arch Neurol Psychiatry 69:171–192
Liepert J, Hamzei F, Weiller C (2000) Motor cortex disinhibition of the unaffected hemisphere after acute stroke. Muscle Nerve 23:1761–1763
Liepert J, Dettmers C, Terborg C, Weiller C (2001) Inhibition of ipsilateral motor cortex during phasic generation of low force. Clin Neurophysiol 112:114–121
Liepert J, Hamzei F, Weiller C (2004) Lesion-induced and training-induced brain reorganization. Restor Neurol Neurosci 22:269–277
Middleton FA, Strick PL (1997) New concepts about the organization of basal ganglia output. Adv Neurol 74:57–68
Middleton FA, Strick PL (2000) Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev 31:236–250
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113
Pascual-Leone A, Peris M, Tormos JM, Pascual AP, Catala MD (1996) Reorganization of human cortical motor output maps following traumatic forearm amputation. Neuroreport 7:2068–2070
Perkel DJ, Farries MA (2000) Complementary ‘bottom-up’ and ‘top-down’ approaches to basal ganglia function. Curr Opin Neurobiol 10:725–731
Pioro EP, Antel JP, Cashman NR, Arnold DL (1994) Detection of cortical neuron loss in motor neuron disease by proton magnetic resonance spectroscopic imaging in vivo. Neurology 44:1933–1938
Price CJ, Friston KJ (1999) Scanning patients with tasks they can perform. Hum Brain Mapp 8:102–108
Roricht S, Machetanz J, Irlbacher K, Niehaus L, Biemer E, Meyer BU (2001) Reorganization of human motor cortex after hand replantation. Ann Neurol 50:240–249
Sanes JN, Donoghue JP (2000) Plasticity and primary motor cortex. Annu Rev Neurosci 23:393–415
Schoenfeld MA, Tempelmann C, Gaul C, Kuhnel GR, Duzel E, Hopf JM, Feistner H, Zierz S, Heinze HJ, Vielhaber S (2005) Functional motor compensation in amyotrophic lateral sclerosis. J Neurol 252(8):944–952
Scholz VH, Flaherty AW, Kraft E, Keltner JR, Kwong KK, Chen YI, Rosen BR, Jenkins BG (2000) Laterality, somatotopy and reproducibility of the basal ganglia and motor cortex during motor tasks. Brain Res 879:204–215
Small SL, Hlustik P, Noll DC, Genovese C, Solodkin A (2002) Cerebellar hemispheric activation ipsilateral to the paretic hand correlates with functional recovery after stroke. Brain 125:1544–1557
Thach WT, Goodkin HP, Keating JG (1992) The cerebellum and the adaptive coordination of movement. Annu Rev Neurosci 15:11–1978–1911.1988
Toni I, Krams M, Turner R, Passingham RE (1998) The time course of changes during motor sequence learning: a whole-brain fMRI study. Neuroimage 8:50–61
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15:273–289
van Mier H, Tempel LW, Perlmutter JS, Raichle ME, Petersen SE (1998) Changes in brain activity during motor learning measured with PET: effects of hand of performance and practice. J Neurophysiol 80:2177–2199
Wall JT, Xu J, Wang X (2002) Human brain plasticity: an emerging view of the multiple substrates and mechanisms that cause cortical changes and related sensory dysfunctions after injuries of sensory inputs from the body. Brain Res Brain Res Rev 39:181–215
Ward NS (2004) Functional reorganization of the cerebral motor system after stroke. Curr Opin Neurol 17:725–730
Ward NS, Brown MM, Thompson AJ, Frackowiak RS (2003a) Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain 126:2476–2496
Ward NS, Brown MM, Thompson AJ, Frackowiak RS (2003b) Neural correlates of outcome after stroke: a cross-sectional fMRI study. Brain 126:1430–1448
Weder B, Seitz RJ (1994) Deficient cerebral activation pattern in stroke recovery. Neuroreport 5:457–460
Weiller C (1998) Imaging recovery from stroke. Exp Brain Res 123:13–17
Weiller C, Chollet F, Friston KJ, Wise RJ, Frackowiak RS (1992) Functional reorganization of the brain in recovery from striatocapsular infarction in man. Ann Neurol 31:463–472
Weiller C, Ramsay SC, Wise RJ, Friston KJ, Frackowiak RS (1993) Individual patterns of functional reorganization in the human cerebral cortex after capsular infarction. Ann Neurol 33:181–189
Wexler BE, Fulbright RK, Lacadie CM, Skudlarski P, Kelz MB, Constable RT, Gore JC (1997) An fMRI study of the human cortical motor system response to increasing functional demands. Magn Reson Imaging 15:385–396
Wiesendanger M, Rouiller EM, Kazennikov O, Perrig S (1996) Is the supplementary motor area a bilaterally organized system? In: Luders HO (ed) Supplementary sensorimotor area. Lippincott-Raven, Philadelphia, pp 85–94
Wunderlich G, Knorr U, Herzog H, Kiwit JC, Freund HJ, Seitz RJ (1998) Precentral glioma location determines the displacement of cortical hand representation. Neurosurgery 42:18–26
Acknowledgements
This work was supported by the NRW-Nachwuchsgruppe Kn2000 of the Nordrhein-Westfalen Ministry of Education and Research (Fö.1KS9604/0), the Interdisciplinary Center of Clinical Research Münster (IZKF Projects FG2, Kne3/074/04, FG4), the Innovative Medizinische Forschung Münster (KN520301), the Deutsche Forschungsgemeinschaft (Kn 285/6-1 and 6-3), the Amyotrophic Lateral Sclerosis Association, and the Muscular Dystrophy Association—ALS Division.
Author information
Authors and Affiliations
Corresponding author
Additional information
Konrad C. and Jansen A. contributed equally to their work
Appendix1
Appendix1
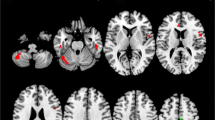
In the present study, cortical activation differences between ALS patients and healthy controls were not as pronounced as previously described. However, the post hoc analysis at more liberal statistical thresholds revealed enhanced activation also in the secondary motor areas (SMA and cingulate motor areas, inferior lateral premotor cortex) of ALS patients in comparison to control subjects (Fig. 4).
Rights and permissions
About this article
Cite this article
Konrad, C., Jansen, A., Henningsen, H. et al. Subcortical reorganization in amyotrophic lateral sclerosis. Exp Brain Res 172, 361–369 (2006). https://doi.org/10.1007/s00221-006-0352-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-006-0352-7