Abstract
The outcome of patients who show no early response to intravenous (i.v.) tissue plasminogen activator (tPA) therapy is poor. The objective of this study was to evaluate the feasibility of rescue localized intra-arterial thrombolysis (LIT) therapy for acute ischemic stroke patients after an early non-responsive i.v. tPA therapy. Patients with proximal MCA occlusions who were treated by LIT (n=10) after failure of early response [no improvement or improvement of National Institute of Health Stroke Scale (NIHSS) scores of ≤3] to i.v. tPA therapy (0.9 mg/kg—10% bolus and 90% i.v. infusion over 60 min) were selected. The recanalization rates, incidence of post-thrombolysis hemorrhage and clinical outcomes [baseline and discharge NIHSS scores, mortality, 3 months Barthel index (BI) and modified Rankin score (mRS)] were evaluated. Rescue LIT therapy was performed on ten MCA occlusion patients (male:female=3:7, mean age 71 years). The mean time between the initiation of i.v. tPA therapy and the initiation of intra-arterial urokinase (i.a. UK) was 117±25.0 min [time to i.v. tPA 137±32 min; time to digital subtraction angiography (DSA) 221±42 min; time to i.a. UK 260±46 min]. The baseline NIHSS scores showed significant improvement at discharge (median from 18 to 6). Symptomatic hemorrhage and, consequent, mortality were noted in 2/10 (20%) patients. Three months good outcome was noted in 4/10 (40%, mRS 0–2) and 3/10 (30%, BI ≥95). In conclusion, rescue LIT therapy can be considered as a treatment option for patients not showing early response to full dose i.v. tPA therapy. Larger scale studies for further validation of this protocol may be necessary.
Similar content being viewed by others
Introduction
The National Institute of Neurological Disorders and Stroke (NINDS) trial has shown that stroke patients treated within 3 h of onset with intravenous (i.v.) recombinant tissue plasminogen activator (tPA) were approximately 30% more likely to exhibit complete or nearly complete recovery at 3 months than were the placebo-treated group. Despite its fast and easy administration protocol and positive effects on the final outcome, approximately 50–60% of the NINDS eligible patients show unfavorable long-term outcome [scores of <95 on the Barthel index (BI) ≥2 on the NIHSS and modified Rankin scale and ≥2 on the Glasgow outcome scale] [1].
Early recanalization has consistently been demonstrated as a powerful predictor of good long-term outcome, but the relationship between the clinical neurological state and the time of recanalization for maximum thrombolytic effect after i.v. tPA therapy is largely unknown [2–5]. Transcranial doppler (TCD) evaluations of the speed of recanalization after administration of i.v. tPA bolus have shown that recanalization usually begins within approximately 30 min and is complete in about 1 h in most cases [2, 3, 6–8]. Persistence of proximal arterial occlusion without signs of early recanalization on TCD after i.v. tPA therapy is reported to be a poor prognostic sign [4–6]. Mortality rates of 39% have been reported for patients not showing early recanalization within 2 h of administration of i.v. tPA bolus [4].
We have adopted a rescue LIT protocol. Patients presenting within 3 h of onset of acute ischemic stroke are treated with full-dose i.v. tPA therapy, and patients showing no early response after the completion of i.v. tPA therapy are treated by rescue LIT therapy.
The purpose of this study was to evaluate the results of our rescue LIT therapy for acute ischemic stroke patients who failed to show good early response to i.v. tPA therapy.
Methods
Patient selection
The patients were selected from a prospectively collected registry of acute ischemic stroke patients. Patients who had been treated with full-dose i.v. tPA (0.9 mg/kg—10% bolus and 90% i.v. infusion over 60 min) within the 3 h window but had shown no early improvement and thus had received additional rescue LIT therapy for proximal MCA occlusion were selected. No early improvement was defined as ‘no improvement or improvement of 3 points or less in NIHSS scores evaluated immediately after the completion of full-dose infusion of i.v. tPA.’ If the patient showed worsening of four points or more in NIHSS scores, an additional CT scan was performed prior to rescue LIT therapy to rule out hemorrhage. Other inclusion criteria included (1) ischemic stroke with a clearly defined time of onset; (2) aged 18 through 85 years; (3) NIHSS score of more than 4 (isolated aphasia or hemianopia cases included in the treatment inclusion criteria); (4) normal or showed early signs on the precontrast brain CT scan without evidence of hemorrhage.
Exclusion criteria included (1) history of symptomatic ischemic stroke within the previous 6 weeks; (2) head trauma (within 90 days); (3) surgery, biopsy from a major organ, recent or active hemorrhage, trauma associated with internal organ injury or an ulcerative wound (within 30 days); (4) arterial puncture at a non-compressible site (within 7 days); (5) seizures at onset; (6) a history of intracranial hemorrhage or a clinical presentation suggestive of a subarachnoid hemorrhage or septic embolism; (7) a systolic blood pressure above 185 mmHg or diastolic blood pressure above 110 mmHg, (8) bleeding diathesis, (9) serious, advanced, or terminal illness; (10) rapidly improving neurological signs; (11) clinical presentation of a lacunar infarction; (12) a comatose state in a suspected carotid arterial stroke. The laboratory exclusion criteria included (a) an increased international normalized ratio greater than 1.7; (b) a history of heparinization within the previous 48 h and an elevated activated partial thromboplastin time; (c) platelet counts <100,000 mm−3; (d) hematocrit <0.4 (25%); (e) blood glucose <2.77 mmol/l (50 mg/dl) or >22.2 mmol/l (400 mg/dl).
The treatment protocol was approved by our institutional review board, and all patients or their representatives signed informed written consent forms.
Thrombolysis procedure
The neuro-intervention team was notified immediately after the initiation of i.v. tPA infusion, and the final decision to perform the rescue LIT therapy procedure was made immediately after the infusion of full-dose i.v. tPA.
Digital subtraction angiography (DSA) was performed by the femoral approach using the Seldinger method. The pre-thrombolysis angiogram protocol included bilateral internal carotid artery and a vertebral artery injection for the purpose of evaluation of collaterals. The LIT therapy was intended in M1 and M2 occlusions. After identification of the proximal MCA occlusion, superselective angiography was performed using a microcatheter. The microcatheter tip was imbedded in the face of the thrombus using an endhole microcatheter (Excelsior, Target Therapeutics, Fremont, Calif., USA) or through the thrombus using a sidehole microcatheter (micro-SOFT STREAM, Target Therapeutics). A dose of 100,000 units of UK was reconstituted in 5 ml of 0.9% normal saline. The UK was infused by hand in a pulsatile injection fashion at a rate of 20,000 units per min using a 1-ml syringe. In most cases, microcatheter angiogram was performed after injection of every 100,000 units of UK to minimize its dosage. The maximum dose of UK was limited to 1,000,000 units. Mechanical thrombolysis with microwire and microcatheter was attempted whenever feasible.
Arterial recanalization was evaluated by the thrombosis in myocardial infarction (TIMI) grade [9]. Arteries showing a TIMI grade 2 or 3 flow were regarded as being recanalized.
In general, additional antithrombotic therapy was initiated 24 h after the LIT therapy. If the cause was considered to be cardiogenic, i.v. heparin therapy was performed. In other cases, oral antiplatelet therapy was performed.
Neuroradiological evaluation
Following the rescue LIT therapy, all patients underwent brain CT or MRI within 24 h. Immediate post-thrombolysis or additional delayed imaging was performed if hemorrhage was clinically suspected. Symptomatic intracranial hemorrhage was defined as neurological worsening ≥4 points in NIHSS score and attributable to the presence of the clot. Three radiologists who were blind to the treatment subgroup and the outcome of the patient analyzed the imaging findings, and the results were reached by consensus.
Outcome measures
The patient’s NIHSS score was obtained on arrival, immediately after i.v. tPA and LIT thrombolysis, at 24 h, at discharge, and at 1 and 3 months after onset of symptoms. The BI was obtained at 3 months after the onset of ischemia. Scores of ≥95 were considered to indicate favorable long-term outcome. The modified Rankin score (mRS) was also used to measure the disability secondary to stroke. A score of 2 or less was considered to indicate a good long-term outcome. One of two neurologists (K.Y.L. or S.H.K.), who were not blind to the angiographic findings, obtained the NIHSS score, the BI and the mRS.
Statistical analysis
The t-test was used to analyze differences between the pre-thrombolysis and post-thrombolysis NIHSS scores. P< 0.05 was considered significant. All values are presented as means ± SD or as medians.
Results
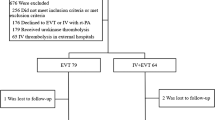
Seventy-four patients underwent i.v. tPA therapy according to the protocol during the study period. Fifty-four patients (73%) showed no remarkable improvement or decrease of NIHSS points of 3 or less. Early response to i.v. tPA therapy was noted in 19 patients (26%), and hemorrhage occurred immediately after i.v. tPA therapy in one patient (1%). Two patients refused further therapy; thus, cerebral angiogram was performed on 52 patients. There were no cases of serious complications associated with the angiographic procedure. Rescue LIT therapy was not performed in 31 patients, owing to: normal angiogram or peripheral branch vessel occlusion (n=14), arterial access failure (n=5), and ICA total occlusion (n=12). Patients who received rescue LIT therapy for proximal MCA occlusions after early non-responsive i.v. tPA therapy (n=10) were selected for analysis in this study.
A summary of the patients’ characteristics is presented in Table 1. The mean age of the patients was 71 years. Cardiac disease (60%), hypertension (30%) and smoking (30%) were the most prominent risk factors.
The initial i.v. tPA therapy was performed within the 3 h time window (mean 137±32 min) in all patients (Table 2). None of the patients showed an increased NIHSS score of more than 4 after the completion of i.v. tPA therapy in this group, thus none was screened by an additional imaging study to rule out hemorrhage before rescue LIT therapy. Additional rescue LIT therapy was performed within the 6 h window (onset to DSA 221±42 min, onset to intra-arterial urokinase (i.a. UK) administration 260±46 min) in all patients who failed to show good early response. The mean time between the initiation of i.v. tPA therapy and the identification of occlusion and initiation of i.a. UK was 117±25.0 min.
The median NIHSS score at admission was 18 (range 10–22). At discharge the median NIHSS score improved to 6 (range 0–10), which was statistically significant (P< 0.05). Successful recanalization was noted in nine of ten (90%) cases after rescue LIT therapy. Symptomatic hemorrhage with neurological deterioration occurred in two of ten (20%) cases; both patients died from hemorrhagic complications.
Good long-term outcome (3 months mRS 0–2, BI ≥95) was observed in 40% (mRS) and 30% (BI) in this rescue therapy group.
Discussion
The results of this study have shown the feasibility of rescue LIT therapy for patients who do not show early response to full dose i.v. tPA therapy [1].
Although recanalization is a significant factor in predicting the outcome of ischemic stroke patients, the relationship between the time point of recanalization from maximum thrombolytic effect after i.v. tPA therapy and the recovery of clinical neurological symptoms is not well known [2–5]. Recent studies using TCD have shown that recanalization usually begins within approximately 30–40 min after the administration of the i.v. tPA bolus and is complete in approximately 1 h in most cases of recanalization [2, 3, 6–8]. The persistence of proximal arterial occlusion without signs of early recanalization on TCD immediately after i.v. tPA therapy is reported to be a poor prognostic sign [4–6]. According to Felberg et al. [5] restoration of flow at the end of the i.v. tPA infusion was a significant factor in differentiating the patients who showed dramatic recovery (dramatic recovery defined as an improvement of ≥10 NIHSS points or a decrease to an NIHSS score ≤3 by the end of full-dose i.v. tPA infusion) and those who did not show dramatic recovery (normal restoration of flow was 58% in dramatic-recovery patients versus 14% in non-dramatic-recovery patients, P< 0.01). Patients experiencing dramatic recovery, probably from the effects of early restoration of flow, also experienced significantly better long-term outcome (median mRS 1 vs 4, P< 0.01).
Labiche et al. [4] also reported that symptomatic hemorrhage and subsequent mortality occurred in 39% of proximal MCA occlusion patients who showed no early recanalization, when monitored with TCD at 2 h after administration of i.v. tPA bolus. Thus, rescue therapy for patients who show no early response to full-dose i.v. tPA therapy can be considered for an additional rescue LIT therapy.
An increased dosage of thrombolytic drugs is known to increase the incidence of hemorrhagic complications [10]. However, our protocol differs from that using an increased single dose of thrombolytics. In our series i.a. UK was sequentially administered approximately 2 h (117±25.0 min) after the i.v. tPA bolus, after identification of the persisting clot, and mechanical thrombolysis was also performed whenever possible to reduce the dose of i.a. UK. In addition, we believe that the combined use of thrombolytics, such as tPA and urokinase type plasminogens, can have synergistic effects on the clot. Such synergistic effects on the coronary angiographic patency and re-occlusion rates in acute myocardial infarct patients have been reported [11–17]. Cumulative fibrinolytic potency or cumulative systemic lytic effect of the drugs, alterations of hemostatic protein variables culminating in fibrinogen depletion, and elevation of fibrinogen degradation products resulting in reduced platelet aggregatibility, have been the proposed mechanisms [12–14, 18, 19].
Combined therapy, such as combined use of i.v. and an i.a. approach and combined use of two or more thrombolytics for acute ischemic stroke patients, has recently gained much interest [20–25]. In the Emergency Management of Stroke (EMS) bridging trial, patients who received i.v. r-tPA (0.6 mg/kg, 60 mg maximum, 10% of the dose as a bolus over 1 min and the remainder over 30 min) followed by local i.a. administration of r-tPA were associated with higher recanalization rates (TIMI 3, 54%; 2, 27%) than those who received i.v. placebo/i.a. r-tPA (TIMI 3, 10%; 2, 40%), although these results were not associated with an improved clinical outcome. Most recently, the results of the Interventional Management of Stroke (IMS) trial (protocol identical to that of the EMS trial except for 15% bolus i.v. tPA) have shown a statistically non-significant but numerically lower mortality rate (16%) than that for placebo (24%) and i.v. tPA-treated subjects (21%) in the NINDS trial. The rate of symptomatic intracerebral hemorrhage (6.3%) in IMS subjects was similar to that of i.v. tPA-treated subjects (6.6%) but was higher than the rate in placebo-treated subjects (1.0%, P=0.018) in the NINDS trial. The IMS subjects had a significantly better outcome at 3 months than placebo-treated subjects but had similar outcome when compared with the i.v. tPA-treated patients in the NINDS trial. These results may seem unimpressive, but the patients in the IMS study were more likely to have had early CT changes in the baseline CT, atrial fibrillation, a higher baseline systolic blood pressure, and to have had i.v. rt-PA begun significantly later (median 140 min), than placebo- (108 min) and rt-PA-treated subjects (90 min) in the NINDS trial.
Our protocol should be distinguished from those reports because we have shown the feasibility of LIT therapy as a ‘rescue’ procedure after clinically unresponsive full-dose i.v. tPA therapy.
Hill et al. [25] reported the results of ‘rescue’ therapy after full-dose i.v. alteplase administration. In their study, six of the eight patients intended for treatment actually received additional LIT therapy. Hill et al. used non-invasive neuroimaging tools for screening after the completion of i.v. thrombolytic therapy. Despite the small number of cases in this series, recanalization (TIMI 2–3) was achieved in two of six (33%) patients. Three of six (50%) patients who were treated with additional i.a. alteplase did well (mRS 0–2), and two of six did extremely well (mRS 0–1) with no major complications. One of the six patients died from contralateral ICA occlusion due to embolus from a left ventricular thrombus.
Our series showed a relatively high rate of symptomatic hemorrhage resulting in mortality (20%). However, the subjects in our group should not be compared equivalently with those in other major trials of LIT therapy. The patients in our study consisted of those who had persistent proximal MCA occlusion despite having received full-dose i.v. tPA therapy. The burden or the component of the thrombi in these patients was more resistant to thrombolytic therapy, and, thus, the patients were probably at higher risk of grave outcome. In spite of these circumstances, recanalization was achieved in 90% of cases, with favorable long-term outcome in terms of 3-month mRS (0–2) and 3-month BI (≥95) in 40% and 30% of the patients, respectively. These results seem compatible to the LIT results of the Prolyse in Acute Cerebral Thromboembolism (PROACT) II trial in which the symptomatic hemorrhage rate and mortality rates were, respectively, 10% and 25%, with favorable long-term outcome in 40% (3-month mRS 0–2) and 41% (3-month BI ≥90), for proximal MCA occlusion patients treated within the 6 h window [26].
The addition of an obligatory screening neuroimaging study before the rescue therapy could have reduced the hemorrhage rate in our study. However, in the study by Hill et al. [25], which included an additional MR study before LIT therapy, the median time from onset to i.v. tPA bolus administration was 101.5 min, but the time to LIT therapy was 310 min. In comparison, the respective median times were 145 min (mean 137±32 min) and 272.5 min (mean 260±46 min) in our study. Performing an additional imaging study can be a source of significant time delay. Thus, considering the benefits of earlier intervention and recanalization, we believe that substituting a clinical neurological examination for an additional imaging study, with the additional imaging study performed only in the case of clinical deterioration (increase of NIHSS scores ≥4), can be considered more beneficial.
In conclusion, rescue LIT therapy can be considered as a treatment option for patients not showing early response to full-dose i.v. tPA therapy. Larger scale studies for further validation of this protocol may be necessary.
References
The National Institute Of Neurological Disorders And Stroke rt-PA Stroke Study Group (1995) Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 333:1581–1587
Christou I, Alexandrov AV, Burgin WS, Wojner AW, Felberg RA, Malkoff M, Grotta JC (2000) Timing of recanalization after tissue plasminogen activator therapy determined by transcranial doppler correlates with clinical recovery from ischemic stroke. Stroke 31:1812–1816
Alexandrov AV, Burgin WS, Demchuk AM, El-Mitwalli A, Grotta JC (2001) Speed of intracranial clot lysis with intravenous tissue plasminogen activator therapy: sonographic classification and short-term improvement. Circulation 103:2897–2902
Labiche LA, Al-Senani F, Wojner AW, Grotta JC, Malkoff M, Alexandrov AV (2003) Is the benefit of early recanalization sustained at 3 months? A prospective cohort study. Stroke 34:695–698
Felberg RA, Okon NJ, El-Mitwalli A, Burgin WS, Grotta JC, Alexandrov AV (2002) Early dramatic recovery during intravenous tissue plasminogen activator infusion: clinical pattern and outcome in acute middle cerebral artery stroke. Stroke 33:1301–1307
Molina CA, Alexandrov AV, Demchuk AM, Saqqur M, Uchino K, Alvarez-Sabin J (2004) Improving the predictive accuracy of recanalization on stroke outcome in patients treated with tissue plasminogen activator. Stroke 35:151–156
Christou I, Burgin WS, Alexandrov AV, Grotta JC (2001) Arterial status after intravenous TPA therapy for ischaemic stroke. A need for further interventions. Int Angiol 20:208–213
Alexandrov AV, Molina CA, Grotta JC, Garami Z, Ford SR, Alvarez-Sabin J, Montaner J, Saqqur M, Demchuk AM, Moye LA, Hill MD, Wojner AW (2004) Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med 351:2170–2178
TIMI Study Group (1985) The thrombolysis in myocardial infarction (TIMI) trial. Phase I findings. N Engl J Med 312:932–936
Brott TG, Haley EC Jr, Levy DE, Barsan W, Broderick J, Sheppard GL, Spilker J, Kongable GL, Massey S, Reed R, et al (1992) Urgent therapy for stroke part I. Pilot study of tissue plasminogen activator administered within 90 min. Stroke 23:632–640
Zarich SW, Kowalchuk GJ, Weaver WD, Loscalzo J, Sassower M, Manzo K, Byrnes C, Muller JE, Gurewich V (1995) Sequential combination thrombolytic therapy for acute myocardial infarction: results of the Pro-Urokinase and t-PA Enhancement of Thrombolysis (PATENT) Trial. J Am Coll Cardiol 26:374–379
Morris JA, Muller DW, Topol EJ (1991) Combination thrombolytic therapy: a comparison of simultaneous and sequential regimens of tissue plasminogen activator and urokinase. Am Heart J 122:375–380
Califf RM, Topol EJ, Stack RS, Ellis SG, George BS, Kereiakes DJ, Samaha JK, Worley SJ, Anderson JL, Harrelson-Woodlief L, et al (1991) Evaluation of combination thrombolytic therapy and timing of cardiac catheterization in acute myocardial infarction. Results of thrombolysis and angioplasty in myocardial infarction—phase five randomized trial. TAMI Study Group. Circulation 83:1543–1556
Popma JJ, Califf RM, Ellis SG, George BS, Kereiakes DJ, Samaha JK, Worley SJ, Anderson JL, Stump D, Woodlief L, et al (1992) Mechanism of benefit of combination thrombolytic therapy for acute myocardial infarction: a quantitative angiographic and hematologic study. J Am Coll Cardiol 20:1305–1312
Topol EJ, Califf RM, George BS, Kereiakes DJ, Rothbaum D, Candela RJ, Abbotsmith CW, Pinkerton CA, Stump DC, Collen D, et al (1988) Coronary arterial thrombolysis with combined infusion of recombinant tissue-type plasminogen activator and urokinase in patients with acute myocardial infarction. Circulation 77:1100–1107
Kidwell CS, Saver JL, Carneado J, Sayre J, Starkman S, Duckwiler G, Gobin YP, Jahan R, Vespa P, Villablanca JP, Liebeskind DS, Vinuela F (2002) Predictors of hemorrhagic transformation in patients receiving intra-arterial thrombolysis. Stroke 33:717–724
Maksimenko AV, Tischenko EG (1999) New thrombolytic strategy: bolus administration of tPA and urokinase–fibrinogen conjugate. J Thromb Thrombolysis 7:307–312
Stachurska J, Latallo Z, Kopec M (1970) Inhibition of platelet aggregation by dialysable fibrinogen degradation products (FDP). Thromb Diath Haemorrh 23:91–98
Stump DC, Califf RM, Topol EJ, Sigmon K, Thornton D, Masek R, Anderson L, Collen D (1989) Pharmacodynamics of thrombolysis with recombinant tissue-type plasminogen activator. Correlation with characteristics of and clinical outcomes in patients with acute myocardial infarction. The TAMI Study Group. Circulation 80:1222–1230
Ernst R, Pancioli A, Tomsick T, Kissela B, Woo D, Kanter D, Jauch E, Carrozzella J, Spilker J, Broderick J (2000) Combined intravenous and intra-arterial recombinant tissue plasminogen activator in acute ischemic stroke. Stroke 31:2552–2557
Keris V, Rudnicka S, Vorona V, Enina G, Tilgale B, Fricbergs J (2001) Combined intraarterial/intravenous thrombolysis for acute ischemic stroke. AJNR Am J Neuroradiol 22:352–358
Suarez JI, Zaidat OO, Sunshine JL, Tarr R, Selman WR, Landis DM (2002) Endovascular administration after intravenous infusion of thrombolytic agents for the treatment of patients with acute ischemic strokes. Neurosurgery 50:251–260
Zaidat OO, Suarez JI, Santillan C, Sunshine JL, Tarr RW, Paras VH, Selman WR, Landis DM (2002) Response to intra-arterial and combined intravenous and intra-arterial thrombolytic therapy in patients with distal internal carotid artery occlusion. Stroke 33:1821–1826
Lewandowski CA, Frankel M, Tomsick TA, Broderick J, Frey J, Clark W, Starkman S, Grotta J, Spilker J, Khoury J, Brott T (1999) Combined intravenous and intra-arterial r-TPA versus intra-arterial therapy of acute ischemic stroke: emergency management of stroke (EMS) Bridging Trial. Stroke 30:2598–2605
Hill MD, Barber PA, Demchuk AM, Newcommon NJ, Cole-Haskayne A, Ryckborst K, Sopher L, Button A, Hu W, Hudon ME, Morrish W, Frayne R, Sevick RJ, Buchan AM (2002) Acute intravenous—intra-arterial revascularization therapy for severe ischemic stroke. Stroke 33:279–282
Furlan A, Higashida R, Wechsler L, Gent M, Rowley H, Kase C, Pessin M, Ahuja A, Callahan F, Clark WM, Silver F, Rivera F (1999) Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA 282:2003–2011
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, D.J., Kim, D.I., Kim, S.H. et al. Rescue localized intra-arterial thrombolysis for hyperacute MCA ischemic stroke patients after early non-responsive intravenous tissue plasminogen activator therapy. Neuroradiology 47, 616–621 (2005). https://doi.org/10.1007/s00234-005-1388-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-005-1388-2