Abstract
Introduction
Susceptibility-weighted imaging (SWI) is a novel magnetic resonance (MR) technique that exploits the magnetic susceptibility differences of various tissues, such as blood, iron and calcification. This pictorial review covers many clinical conditions illustrating its usefulness.
Methods
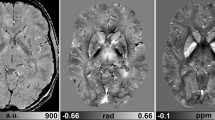
SWI consists of using both magnitude and phase images from a high-resolution, three-dimensional fully velocity-compensated gradient echo sequence. Phase mask is created from the MR phase images, and multiplying these with the magnitude images increase the conspicuity of the smaller veins and other sources of susceptibility effects, which is depicted using minimal intensity projection (minIP).
Results
The phase images are useful in differentiating between diamagnetic and paramagnetic susceptibility effects of calcium and blood, respectively. This unique MR sequence will help in detecting occult low flow vascular lesions, calcification and cerebral microbleed in various pathologic conditions and aids in characterizing tumors and degenerative diseases of the brain. This sequence also can be used to visualize normal brain structures with conspicuity.
Conclusion
Susceptibility-weighted imaging is useful in differentiating and characterizing diverse brain pathologies.

















Similar content being viewed by others
References
Reichenbach JR, Venkatesan R, Schillinger DJ et al (1997) Small vessels in the human brain. MR venography with deoxyhemoglobin as an intrinsic contrast agent. Radiology 204:272–277
Reichenbach JR, Haacke EM (2001) High-resolution BOLD venographic imaging: a window into brain function. NMR Biomed 14:453–467
Seghal V, Delproposto Z, Haacke EM et al (2005) Clinical application s of neuroimaging with susceptibility-weighted imaging. J Magn Reson Imaging 22:439–450
Liang L, Korogi Y, Sugahara T, Shigematsu Y, Okuda T, Ikushima I, Takahashi M (1999) Detection of intracranial hemorrhage with susceptibility-weighted MR sequences. AJNR Am J Neuroradiol 20:1527–1534
Wang Y, Yu Y, Li D et al (2000) Artery and vein separation using susceptibility-dependent phase in contrast enhanced MRA. J Magn Reson Imaging 12:661–670
Rauscher A, Sedlacik J, Barth M, Haacke EM, Reichenbach JR et al (2005) Magnetic susceptibility-weighted MR phase imaging of the human brain. AJNR Am J Neroradiol 26:736–742
Yamada N, Imakita S, Sakuma T et al (1996) Intracranial calcification on gradient -echo phase image:Depiction of Diamagnetic susceptibility. Radiology 198:171–178
Gupta RK, Rao SB, Rajan J et al (2001) Differentiation of calcification from chronic hemorrhage with corrected gradient echo phase imaging. J Comput Assist Tomogr 25:698–704
Yamada N, Imakita S, Sakuma T et al (1990) Evaluation of the susceptibility effect on the phase images of a simple gradient echo. Radiology 175:561–565
Deistung A, Mentzel HJ, Rauscher A, Witoszynskyj S, Kaiser WA, Reichenbach JR (2006) Demonstration of paramagnetic and diamagnetic cerebral lesions by using susceptibility weighted phase imaging (SWI). Z Med Phys 16:261–267
Dumoulin CL, Hart HJ (1986) Magnetic resonance angiography. Radiology 161:717–720
Thulborn KR, Waterton JC, Matthews PM et al (1982) Oxygenation dependence of the transverse relaxation time of water protons in whole blood at high field. Biochim Biophys Acta 714:265–270
Li D, Waight DJ, Wang Y (1998) In vivo correlation between blood T2* and oxygen saturation. J Magn Reson Imaging 8:1236–1239
Gomori JM, Grossman RI, Yu-Ip C et al (1987) NMR relaxation times of blood: dependence on field strength, oxidation state, and cell integrity. J Comput Assist Tomogr 11:684–690
Haacke EM, Satin W (1993) Fast imaging and vessel contrast. In: Potchen EJ, Haacke EM, Siebert JE, Gottaschalk A (eds) Magneticresonanceangiography. Mosby, St. Louis, pp 46–51
Reichenbach JR, Venkatesan R, Yablonsky DA et al (1997) Theory and application of static field inhomogenity effects inn gradient echo imaging. J Magn Reson Imaging 7:266–279
Lee BCP, Vo KD, Kido DK et al (1999) MR high resolution blood oxygenation level dependent venography of occult (low-flow) vascular lesions. AJNR Am J Neuroradiol 20:1239–1242
Reichenbach JR, Jonetz-Mentzel L, Fitzek C et al (2001) High resolution blood oxygen-level dependent MR venography. Neuroradiology 43:364–369
Essig M, Reichenbach JR, Schad JR et al (1999) High resolution MR venography of cerebral arteriovenous malformations. Magn Reson Imaging 17:1417–1425
Lin W, Mukherjee P, An H et al (1999) Improving high resolution MR bold venographic imaging using a T1 reducing contrast agent. J Magn Reson Imaging 10:118–123
Dean Bl, Drayer BP, Bird CR, Flora RA, Hodak JA, Coons SW, Carey RG (1990) Gliomas:classification with MR imaging. Radiology 174:411–415
Ding B, Ling HW, Chen KM, Jiang H, Zhu YB (2006) Comparison of cerebral blood volume and permeability in preoperative grading of intracranial glioma using CT perfusion imaging. Neuroradiology 48:773–781
Recht L, Torres CO, Smith TW et al (1990) Transferrin receptor in normal and neoplastic brain tissue :implications for brain tumor immunotherapy. J Neurosurg 72:941–945
Bagley L, Grossman RI, Judy KD et al (1997) Gliomas: correlation of magnetic susceptibility artifact with histologic grade. Radiology 202:511–516
Seghal V, Delproposto Z, Haddar D et al (2006) Susceptibility-weighted imaging to visualise blood products and improve tumor contrast in the study of brain masses. J Magn Reson Imaging 24:41–51
Christoforidis GA, Kangarlu A, Abduljalil AM et al (2004) Susceptibility-based imaging of glioblastoma Microvascularity at 8T.: correlation of MR imaging and postmortem pathology. AJNR Am J Neuroradiol 25:756–760
Krishnamoorthy T, Radhakrishnan VV, Thomas B et al (2006) Microhemorrhages in vestibular schwannomas: prospective study with T2* weighted gradient-echo sequence. In: Proc Am Soc Neurorad (ASNR). San Diego, USA
Wardlaw JM, Statham PF (2000) How often is haemosiderin not visible on routine MRI following traumatic intracerebral haemorrhage? Neuroradiology 42:81–84
Gentry LR, Godersky JC, Thompsonn B (1988) MR imaging of head trauma:review of the distribution and radiopathologic features of traumatic lesions. AJR Am J Roentgenol 150:663–672
Medana IM, Esiri MM (2003) Axonal damage: a key predictor of outcome in human CNS diseases. Brain 126:515–530
Orrison WW, Gentry LR, Stimac GK et al (1994) Blinded comparison of cranial CT and MR in closed head injury evaluation. AJNR Am J Neuroradiology 15:351–356
Paterakis K, Karantanas AH, Komnos A et al (2000) Outcome of patients with diffuse axonal injury: the significance and prognostic value of MRI in the acute phase. J Trauma 49:1071–1075
Atlas SW, Mark AS, Grossman RI et al (1988) Intracranial Hemorrhage: gradient echo MR imaging at 1.5 T.-comparison with spin echo imaging and clinical applications. Radiology 168:803–807
Tong KA, Ashwal S, Holshouer B et al (2003) Hemorrhagic shearing lesions in children and adolescents with posttraumatic diffuse axonal injury: improved detection and initial results. Radiology 227:332–339
Wycliffe ND, Chose J, Holshouser B et al (2004) Reliability in detection of hemorrhage in acute stroke by a new three-dimensional gradient recalled echo susceptibility-weighted imaging technique compared to computed tomography: a retrospective study. J Magn Reson Imaging 20:372–377
Mammen EF (1992) Pathogenesis of venous thrombosis. Chest 102:640s–644s
Kidwell CS, Saver JL, Villablanca JP et al (2002) Magnetic resonance imaging detection of microbleeds before thrombolysis:an emerging application. Stroke 33:95–98
Greenberg SM, O’Donnel HC, Schaefer PW et al (1999) MRI detection of new hemorrhages:potential marker of progression in cerebral amyloid angiopathy. Neurology 53:1135–1138
Lesnik OAJ, Van Den Boom R, Van buchem A et al (2001) Cerebral microbleeds in CADASIL. Neurology 57:1066–1070
Martin D, Makus H, Jurgen H et al (2002) Cerebral microbleeds in CADASIL: a gradient echo MRI and autopsy study. Stroke 33:67–71
Campi A, Benndorf G, Filippi M, Reganati P, Martinelli V, Terreni MR (2001) Primary angiitis of the central nervous system: serial MRI of brain and spinal cord. Neuroradiology 43:599–607
Gerrnberg SM, Finklestein SP, Schaefer et al (1996) Petechial hemorrhages accompanying lobar hemorrhage:detection by gradient echo MRI. Neurology 46:1751–1754
Bartzokis G, Tishler TA (2000) MRI evaluation of basal ganglia ferritin iron and neurotoxicity in Alzheimers and huntingtons disease. Cell Mol Biol 46:821–833
Bartzokis G, Cummins J, Markham Ch et al (1999) MRI evaluation of brain iron in earlier and later onset Parkinsons disease and normal subjects. Magn Reson Imaging 17:213–222
Hecht MJ, Fellner C, Schmid A, Neundorfer B, Fellner FA (2005) Cortical T2 signal shortening in amyotrophic lateral sclerosis is not due to iron deposits. Neuroradiology 47:805–808
Vymazal J, Righini A, Brooks RA et al (1999) T1 and T2 in the brain of healthy subjects,patients with Parkinson disease and patients with multiple system atrophy: relation to iron content. Radiology 211:489–495
Ogg RJ, Langston JW, Haacke EM et al (1999) The correlation between phase shifts in gradient-echo MR images and regional brain iron concentration. Magn Reson Imaging 17:1141–1148
Haacke EM, Ayaz M, Khan A, Manova ES, Krishnamurthy B, Gollapalli L, Ciulla C, Kim I, Petersen F, Kirsch W (2007) Establishing a baseline phase behavior in magnetic resonance imaging to determine normal vs. abnormal iron content in the brain. J Magn Reson Imaging 26:256–264
Tan IL, van Schijndel RA, Pouwels PJ, van Walderveen MA, Reichenbach JR, Manoliu RA, Barkhof F (2000) MR venography of multiple sclerosis. AJNR Am J Neuroradiol 21:1039–1042
Reichenbach JR, Barth M, Haacke M et al (2000) High-resolution MR venography at 3.0 tesla. J Comput Assist Tomogr 24:949–957
Acknowledgements
We thank Siemens Medical Systems for providing with the SWI sequence and the post-processing tools. We also thank the Director, SCTIMST, for the permission to publish this paper.
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thomas, B., Somasundaram, S., Thamburaj, K. et al. Clinical applications of susceptibility weighted MR imaging of the brain – a pictorial review. Neuroradiology 50, 105–116 (2008). https://doi.org/10.1007/s00234-007-0316-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-007-0316-z