Abstract
Introduction
Optic neuritis (ON) and any other early manifestation of multiple sclerosis (MS) are referred to as clinically isolated syndrome (CIS) as long as MS is suspected. In this prospective study we aimed to determine whether diffusion tensor imaging (DTI) could quantify structural changes in patients with early MS.
Methods
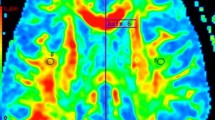
A total of 24 patients and 15 control subjects were prospectively followed by clinical examinations and MRI. the main inclusion criterion was presentation with ON. Patients underwent serial MRI scans: MRI1 (baseline, n=24), MRI2 (mean 6.6 months, n=24), MRI3 (mean 13.0 months, n=14), MRI4 (mean 39.4 months, n=5). Apparent diffusion coefficient (ADC) and fractional anisotropy (FA) maps were derived from DTI. Four regions of interest (ROIs) were defined in normal-appearing white matter (NAWM).
Results
In the temporal course FA decreased in the genu of the callosal body (GCC) from MRI1 to MRI4 (P=0.005) and in the splenium of the callosal body (SCC) (P=0.006). Patients already had lower FA values in the SCC (P<0.01) on MRI1 compared with the controls. Patients had lower FA values in the GCC (P<0.01) starting from MRI2. Patients with definite MS on follow-up (n=9) showed a correlation between FA in the SCC and time (r=−0.40, P=0.004), whereas patients without progression did not.
Conclusions
Our findings suggest that the corpus callosum is an early site for development of anisotropy changes in MS patients with ON. There seems to be a primary FA decrease in all patients with ON that only deteriorates in the group developing definite MS.




Similar content being viewed by others
References
Miller D, Barkhof F, Montalban X, Thompson A, Filippi M (2005) Clinically isolated syndromes suggestive of multiple sclerosis, part 2: non-conventional MRI, recovery processes, and management. Lancet Neurol 4:341–348
Brex PA, Ciccarelli O, O’Riordan JI, Sailer M, Thompson AJ, Miller DH (2002) A longitudinal study of abnormalities on MRI and disability from multiple sclerosis. N Engl J Med 346:158–164
Miller D, Barkhof F, Montalban X, Thompson A, Filippi M (2005) Clinically isolated syndromes suggestive of multiple sclerosis, part I: natural history, pathogenesis, diagnosis, and prognosis. Lancet Neurol 4:281–288
Barkhof F, Filippi M, Miller DH, Scheltens P, Campi A, Polman CH, Comi G, Adèr HJ, Losseff N, Valk J (1997) Comparison of MRI criteria at first presentation to predict conversion to clinically definite multiple sclerosis. Brain 120(Pt 11):2059–2069
Cañellas AR, Gols AR, Izquierdo JR, Subirana MT, Gairin XM (2007) Idiopathic inflammatory-demyelinating diseases of the central nervous system. Neuroradiology 49:393–409
Barkhof F (2002) The clinico-radiological paradox in multiple sclerosis revisited. Curr Opin Neurol 15:239–245
Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mörk S, Bö L (1998) Axonal transection in the lesions of multiple sclerosis. N Engl J Med 338:278–285
Wattjes MP, Harzheim M, Lutterbey GG, Bogdanow M, Schmidt S, Schild HH, Träber F (2008) Prognostic value of high-field proton magnetic resonance spectroscopy in patients presenting with clinically isolated syndromes suggestive of multiple sclerosis. Neuroradiology 50:123–129
Filippi M, Rocca MA (2007) Magnetic resonance imaging techniques to define and monitor tissue damage and repair in multiple sclerosis. J Neurol 254 [Suppl 1]:155–162
Evangelou N, Esiri MM, Smith S, Palace J, Matthews PM (2000) Quantitative pathological evidence for axonal loss in normal appearing white matter in multiple sclerosis. Ann Neurol 47:391–395
Evangelou N, Konz D, Esiri MM, Smith S, Palace J, Matthews PM (2000) Regional axonal loss in the corpus callosum correlates with cerebral white matter lesion volume and distribution in multiple sclerosis. Brain 123(Pt 9):1845–1849
Ge Y, Law M, Johnson G, Herbert J, Babb JS, Mannon LJ, Grossman RI (2004) Preferential occult injury of corpus callosum in multiple sclerosis measured by diffusion tensor imaging. J Magn Reson Imaging 20:1–7
Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, Lublin FD, Metz LM, McFarland HF, O’Connor PW, Sandberg-Wollheim M, Thompson AJ, Weinshenker BG, Wolinsky JS (2005) Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol 58:840–846
Ding XQ, Kucinski T, Wittkugel O, Goebell E, Grzyska U, Görg M, Kohlschütter A, Zeumer H (2004) Normal brain maturation characterized with age-related T2 relaxation times: an attempt to develop a quantitative imaging measure for clinical use. Invest Radiol 39:740–746
Caramia F, Pantano P, Di Legge S, Piattella MC, Lenzi D, Paolillo A, Nucciarelli W, Lenzi GL, Bozzao L, Pozzilli C (2002) A longitudinal study of MR diffusion changes in normal appearing white matter of patients with early multiple sclerosis. Magn Reson Imaging 20:383–388
Gallo A, Rovaris M, Riva R, Ghezzi A, Benedetti B, Martinelli V, Falini A, Comi G, Filippi M (2005) Diffusion-tensor magnetic resonance imaging detects normal-appearing white matter damage unrelated to short-term disease activity in patients at the earliest clinical stage of multiple sclerosis. Arch Neurol 62:803–808
Yu CS, Lin FC, Li KC, Jiang TZ, Zhu CZ, Qin W, Sun H, Chan P (2006) Diffusion tensor imaging in the assessment of normal-appearing brain tissue damage in relapsing neuromyelitis optica. AJNR Am J Neuroradiol 27:1009–1015
Yu CS, Zhu CZ, Li KC, Xuan Y, Qin W, Sun H, Chan P (2007) Relapsing neuromyelitis optica and relapsing-remitting multiple sclerosis: differentiation at diffusion-tensor MR imaging of corpus callosum. Radiology 244:249–256
Pierpaoli C, Basser PJ (1996) Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med 36:893–906
Basser PJ, Pierpaoli C (1996) Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B 111:209–219
Ferguson B, Matyszak MK, Esiri MM, Perry VH (1997) Axonal damage in acute multiple sclerosis lesions. Brain 120(Pt 3):393–399
Allen IV, McKeown SR (1979) A histological, histochemical and biochemical study of the macroscopically normal white matter in multiple sclerosis. J Neurol Sci 41:81–91
Filippi M, Rocca MA, Falini A, Caputo D, Ghezzi A, Colombo B, Scotti G, Comi G (2002) Correlations between structural CNS damage and functional MRI changes in primary progressive MS. Neuroimage 15:537–546
Cercignani M, Bozzali M, Iannucci G, Comi G, Filippi M (2002) Intra-voxel and inter-voxel coherence in patients with multiple sclerosis assessed using diffusion tensor MRI. J Neurol 249:875–883
Rovaris M, Iannucci G, Falautano M, Possa F, Martinelli V, Comi G, Filippi M (2002) Cognitive dysfunction in patients with mildly disabling relapsing-remitting multiple sclerosis: an exploratory study with diffusion tensor MR imaging. J Neurol Sci 195:27–30
Tortorella C, Viti B, Bozzali M, Sormani MP, Rizzo G, Gilardi MF, Comi G, Filippi M (2000) A magnetization transfer histogram study of normal-appearing brain tissue in MS. Neurology 54:186–193
Pagani E, Filippi M, Rocca MA, Horsfield MA (2005) A method for obtaining tract-specific diffusion tensor MRI measurements in the presence of disease: application to patients with clinically isolated syndromes suggestive of multiple sclerosis. Neuroimage 26:258–265
Griffin CM, Chard DT, Ciccarelli O, Kapoor B, Barker GJ, Thompson AI, Miller DH (2001) Diffusion tensor imaging in early relapsing-remitting multiple sclerosis. Mult Scler 7:290–297
Chepuri NB, Yen YF, Burdette JH, Li H, Moody DM, Maldjian JA (2002) Diffusion anisotropy in the corpus callosum. AJNR Am J Neuroradiol 23:803–808
Witelson SF (1989) Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain 112(Pt 3):799–835
Abe O, Aoki S, Hayashi N, Yamada H, Kunimatsu A, Mori H, Yoshikawa T, Okubo T, Ohtomo K (2002) Normal aging in the central nervous system: quantitative MR diffusion-tensor analysis. Neurobiol Aging 23:433–441
Pelletier J, Habib M, Lyon-Caen O, Salamon G, Poncet M, Khalil R (1993) Functional and magnetic resonance imaging correlates of callosal involvement in multiple sclerosis. Arch Neurol 50:1077–1082
Ceccarelli A, Rocca MA, Falini A, Tortorella P, Pagani E, Rodegher M, Comi G, Scotti G, Filippi M (2007) Normal-appearing white and grey matter damage in MS: a volumetric and diffusion tensor MRI study at 3.0 Tesla. J Neurol 254:513–518
Levin HS, Benavidez DA, Verger-Maestre K, Perachio N, Song J, Mendelsohn DB, Fletcher JM (2000) Reduction of corpus callosum growth after severe traumatic brain injury in children. Neurology 54:647–653
Foong J, Maier M, Clark CA, Barker GJ, Miller DH, Ron MA (2000) Neuropathological abnormalities of the corpus callosum in schizophrenia: a diffusion tensor imaging study. J Neurol Neurosurg Psychiatr 68:242–244
Gallucci M, Limbucci N, Paonessa A, Caranci F (2007) Reversible focal splenial lesions. Neuroradiology 49:541–544
Bjartmar C, Wujek JR, Trapp BD (2003) Axonal loss in the pathology of MS: consequences for understanding the progressive phase of the disease. J Neurol Sci 206:165–171
Medana IM, Esiri MM (2003) Axonal damage: a key predictor of outcome in human CNS diseases. Brain 126:515–530
Ciccarelli O, Werring DJ, Barker GJ, Griffin CM, Wheeler-Kingshott CA, Miller DH, Thompson AJ (2003) A study of the mechanisms of normal-appearing white matter damage in multiple sclerosis using diffusion tensor imaging – evidence of Wallerian degeneration. J Neurol 250:287–292
Simon JH, Zhang S, Laidlaw DH, Miller DE, Brown M, Corboy J, Bennett J (2006) Identification of fibers at risk for degeneration by diffusion tractography in patients at high risk for MS after a clinically isolated syndrome. J Magn Reson Imaging 24:983–988
Ranjeva JP, Pelletier J, Confort-Gouny S, Ibarrola D, Audoin B, Le Fur Y, Viout P, Chérif AA, Cozzone PJ (2003) MRI/MRS of corpus callosum in patients with clinically isolated syndrome suggestive of multiple sclerosis. Mult Scler 9:554–565
Hasan KM, Gupta RK, Santos RM, Wolinsky JS, Narayana PA (2005) Diffusion tensor fractional anisotropy of the normal-appearing seven segments of the corpus callosum in healthy adults and relapsing-remitting multiple sclerosis patients. J Magn Reson Imaging 21:735–743
Coombs BD, Best A, Brown MS, Miller DE, Corboy J, Baier M, Simon JH (2004) Multiple sclerosis pathology in the normal and abnormal appearing white matter of the corpus callosum by diffusion tensor imaging. Mult Scler 10:392397
Bammer R, Augustin M, Strasser-Fuchs S, Seifert T, Kapeller P, Stollberger R, Ebner F, Hartung HP, Fazekas F (2000) Magnetic resonance diffusion tensor imaging for characterizing diffuse and focal white matter abnormalities in multiple sclerosis. Magn Reson Med 44:583–591
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00234-008-0452-0
Rights and permissions
About this article
Cite this article
Bester, M., Heesen, C., Schippling, S. et al. Early anisotropy changes in the corpus callosum of patients with optic neuritis. Neuroradiology 50, 549–557 (2008). https://doi.org/10.1007/s00234-008-0377-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-008-0377-7