Abstract
Introduction
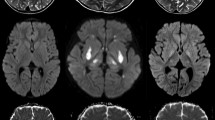
Severe neurological symptoms in Shiga toxin-producing Escherichia coli infection associated hemolytic–uremic syndrome (STEC–HUS) are often accompanied by none or only mild alterations of cerebral magnetic resonance imaging (MRI). This study aims to analyze if quantitative MRI is able to reveal cerebral pathological alterations invisible for conventional MRI.
Methods
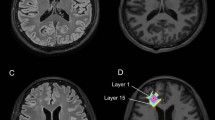
In nine patients with STEC–HUS associated severe neurological symptoms but inconspicuous cerebral MRI findings maps of the parameters T2 relaxation time, relative proton density (PD), apparent diffusion coefficient (ADC), and fractional anisotropy (FA) were generated. Quantitative values of these parameters were measured at the basal ganglia, thalamus, and white matter of the frontal and parietal lobe and compared to those of nine age- and sex-matched controls.
Results
Significant T2 prolongation (p < 0.01) was found in the basal ganglia of all patients compared to controls. PD and ADC were not significantly altered. A significant reduction of FA in patients was seen at caput nuclei caudati (p < 0.01).
Conclusion
Prolonged T2 relaxation time indicates cerebral microstructural damages in these patients despite their inconspicuous MRI findings. T2 relaxometry could be used as a complementary tool for the assessment of metabolic–toxic brain syndromes.




Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- FA:
-
Fractional anisotropy
- MRI:
-
Magnetic resonance imaging
- PD:
-
Proton density
- STEC:
-
Shiga toxin-producing E. coli
- STEC–HUS:
-
Shiga toxin-producing E. coli infection associated hemolytic–uremic syndrome
- Stx:
-
Shiga toxin
References
Roche-Martinez A, Poo P, Maristany-Cucurella M, Jimenez-Llort A, Camacho JA, Campistol J (2008) Neurologic presentation in haemolytic–uraemic syndrome. Rev Neurol 47(4):191–196
Eriksson KJ, Boyd SG, Tasker RC (2001) Acute neurology and neurophysiology of haemolytic–uraemic syndrome. Arch Dis Child 84(5):434–435
Gallo EG, Gianantonio CA (1995) Extrarenal involvement in diarrhoea-associated haemolytic–uraemic syndrome. Pediatr Nephrol 9(1):117–119
Keir L, Coward RJ (2011) Advances in our understanding of the pathogenesis of glomerular thrombotic microangiopathy. Pediatrics 26(4):523–533, Epub 2010
Zoja C, Buelli S, Morigi M (2010) Shiga toxin-associated hemolytic uremic syndrome: pathophysiology of endothelial dysfunction. Pediatrics 25(11):2231–2240, Epub 2010 Apr 2228
Bale JF Jr, Brasher C, Siegler RL (1980) CNS manifestations of the hemolytic–uremic syndrome. Relationship to metabolic alterations and prognosis. Am J Dis Child 134(9):869–872
Obata F (2010) Influence of Escherichia coli shiga toxin on the mammalian central nervous system. Adv Appl Microbiol 71:1–19
Magnus T, Röther J, Simova O, Meier-Cillien M, Repenthin J, Möller F, Gbadamosi J, Panzer U, Wengenroth M, Hagel C, Kluge S, Stahl RK, Wegscheider K, Urban P, Eckert B, Glatzel M, Fiehler J, Gerloff C (2012) The neurological syndrome in adults during the 2011 northern German E. coli serotype O104:H4 outbreak. Brain 135(Pt 6):1850–1859
Weissenborn K, Donnerstag F, Kielstein JT, Heeren M, Worthmann H, Hecker H, Schmitt R, Schiffer M, Pasedag T, Schuppner R, Tryc AB, Raab P, Hartmann H, Ding XQ, Hafer C, Menne J, Schmidt BM, Bultmann E, Haller H, Dengler R, Lanfermann H, Giesemann AM (2012) Neurologic manifestations of E. coli infection-induced hemolytic–uremic syndrome in adults. Neurology 79(14):1466–1473
Upadhyaya K, Barwick K, Fishaut M, Kashgarian M, Siegel NJ (1980) The importance of nonrenal involvement in hemolytic–uremic syndrome. Pediatrics 65(1):115–120
Steinborn M, Leiz S, Rudisser K, Griebel M, Harder T, Hahn H (2004) CT and MRI in haemolytic uraemic syndrome with central nervous system involvement: distribution of lesions and prognostic value of imaging findings. Pediatr Radiol 34(10):805–810
Ohlmann D, Hamann GF, Hassler M, Schimrigk K (1996) Involvement of the central nervous system in hemolytic uremic syndrome/thrombotic thrombocytopenic purpura. Nervenarzt 67(10):880–882
Ding XQ, Sun Y, Kruse B, Illies T, Zeumer H, Fiehler J, Lanfermann H (2009) Microstructural callosal abnormalities in normal-appearing brain of children with developmental delay detected with diffusion tensor imaging. Eur Radiol 19(6):1537–1543
Ding XQ, Fiehler J, Kohlschutter B, Wittkugel O, Grzyska U, Zeumer H, Ullrich K (2008) MRI abnormalities in normal-appearing brain tissue of treated adult PKU patients. J Magn Reson Imaging 27(5):998–1004
Ding XQ, Wittkugel O, Goebell E, Förster AF, Grzyska U, Zeumer H, Fiehler J (2008) Clinical applications of quantitative T2 determination: a complementary MRI tool for routine diagnosis of suspected myelination disorders. Eur J Paediatr Neurol 12(4):298–308, Epub 2007 Oct
Leppert IR, Almli CR, McKinstry RC, Mulkern RV, Pierpaoli C, Rivkin MJ, Pike GB (2009) T(2) relaxometry of normal pediatric brain development. J Magn Reson Imaging 29(2):258–267
Abramoff MD, Magelhaes PJ, Ram SJ (2004) Image processing with ImageJ. Biophoton Int 11(7):36–42
Nathanson S, Kwon T, Elmaleh M, Charbit M, Launay EA, Harambat J, Brun M, Ranchin B, Bandin F, Cloarec S, Bourdat-Michel G, Piètrement C, Champion G, Ulinski T, Deschênes G (2010) Acute neurological involvement in diarrhea-associated hemolytic uremic syndrome. Clin J Am Soc Nephrol 5(7):1218–1228
Donnerstag F, Ding X, Pape L, Bültmann E, Lücke T, Zajaczek J, Hoy L, Das AM, Lanfermann H, Ehrich J, Hartmann H (2012) Patterns in early diffusion-weighted MRI in children with haemolytic uraemic syndrome and CNS involvement. Eur Radiol 22(3):506–513
Ono J, Kodaka R, Imai K, Itagaki Y, Tanaka J, Inui K, Nagai T, Sakurai K, Harada K, Okada S (1993) Evaluation of myelination by means of the T2 value on magnetic resonance imaging. Brain Dev 15(6):433–438
Ding XQ, Kucinski T, Wittkugel O, Goebell E, Grzyska U, Görg M, Kohlschütter A, Zeumer H (2004) Normal brain maturation characterized with age-related T2 relaxation times: an attempt to develop a quantitative imaging measure for clinical use. Invest Radiol 39(12):740–746
Bick U, Ullrich K, Stöber U, Möller H, Schuierer G, Ludolph AC, Oberwittler C, Weglage J, Wendel U (1993) White matter abnormalities in patients with treated hyperphenylalaninaemia: magnetic resonance relaxometry and proton spectroscopy findings. Eur J Pediatr 152(12):1012–1020
Larocque MP, Syme A, Yahya A, Wachowicz K, Allalunis-Turner J, Fallone BG (2010) Monitoring T2 and ADC at 9.4 T following fractionated external beam radiation therapy in a mouse model. Phys Med Biol 55(5):1381–1393
Cieszanowski A, Anysz-Grodzicka A, Szeszkowski W, Kaczynski B, Maj E, Gornicka B, Grodzicki M, Grudzinski IP, Stadnik A, Krawczyk M, Rowinski O (2012) Characterization of focal liver lesions using quantitative techniques: comparison of apparent diffusion coefficient values and T2 relaxation times. Eur Radiol 22(11):2514–2524
Madler B, Drabycz SA, Kolind SH, Whittall KP, MacKay AL (2008) Is diffusion anisotropy an accurate monitor of myelination? Correlation of multicomponent T2 relaxation and diffusion tensor anisotropy in human brain. Magn Reson Imaging 26(7):874–888
Bauwens A, Bielaszewska M, Kemper B, Langehanenberg P, von Bally G, Reichelt R, Mulac D, Humpf HU, Friedrich AW, Kim KS, Karch H, Müthing J (2011) Differential cytotoxic actions of Shiga toxin 1 and Shiga toxin 2 on microvascular and macrovascular endothelial cells. Thromb Haemost 105(3):515–528
Takahashi K, Funata N, Ikuta F, Sato S (2008) Neuronal apoptosis and inflammatory responses in the central nervous system of a rabbit treated with Shiga toxin-2. J Neuroinflammation 5:11
Goldstein J, Loidl CF, Creydt VP, Boccoli J, Ibarra C (2007) Intracerebroventricular administration of Shiga toxin type 2 induces striatal neuronal death and glial alterations: an ultrastructural study. Brain Res 1161:106–115
Tironi-Farinati C, Loidl CF, Boccoli J, Parma Y, Fernandez-Miyakawa ME, Goldstein J (2010) Intracerebroventricular Shiga toxin 2 increases the expression of its receptor globotriaosylceramide and causes dendritic abnormalities. J Neuroimmunol 222(1-2):48–61
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Weissenborn, K., Bültmann, E., Donnerstag, F. et al. Quantitative MRI shows cerebral microstructural damage in hemolytic–uremic syndrome patients with severe neurological symptoms but no changes in conventional MRI. Neuroradiology 55, 819–825 (2013). https://doi.org/10.1007/s00234-013-1176-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-013-1176-3