Abstract
Introduction
Perfusion magnetic resonance imaging (MRI) can be used in the pre-operative assessment of brain tumours. The aim of this prospective study was to identify the perfusion parameters from dynamic contrast-enhanced (DCE) and dynamic susceptibility contrast (DSC) perfusion imaging that could best discriminate between grade II and III gliomas.
Methods
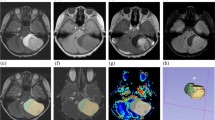
MRI (3 T) including morphological ((T2 fluid attenuated inversion recovery (FLAIR) and T1-weighted (T1W)+Gd)) and perfusion (DCE and DSC) sequences was performed in 39 patients with newly diagnosed suspected low-grade glioma after written informed consent in this review board-approved study. Regions of interests (ROIs) in tumour area were delineated on FLAIR images co-registered to DCE and DSC, respectively, in 25 patients with histopathological grade II (n = 18) and III (n = 7) gliomas. Statistical analysis of differences between grade II and grade III gliomas in histogram perfusion parameters was performed, and the areas under the curves (AUC) from the ROC analyses were evaluated.
Results
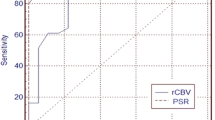
In DCE, the skewness of transfer constant (k trans) was found superior for differentiating grade II from grade III in all gliomas (AUC 0.76). In DSC, the standard deviation of relative cerebral blood flow (rCBF) was found superior for differentiating grade II from grade III gliomas (AUC 0.80).
Conclusions
Histogram parameters from k trans (DCE) and rCBF (DSC) could most efficiently discriminate between grade II and grade III gliomas.


Similar content being viewed by others
Abbreviations
- AUC:
-
Area under the curve
- DCE:
-
Dynamic contrast-enhanced
- DSC:
-
Dynamic susceptibility contrast
- FLAIR:
-
Fluid attenuated inversion recovery
- ICA:
-
Internal carotid artery
- k trans :
-
Transfer constant
- K app :
-
Apparent transfer constant
- ROI:
-
Region of interest
- ROC:
-
Receiver operating characteristics
- T1W:
-
T1-weighted
- TE:
-
Echo time
- TR:
-
Repetition time
References
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114(2):97–109. doi:10.1007/s00401-007-0243-4
Hartmann C, von Deimling A (2009) Molecular pathology of oligodendroglial tumors. Recent Results Cancer Res 171:25–49. doi:10.1007/1978-1003-1540-31206-31202_31202
Walker C, Baborie A, Crooks D, Wilkins S, Jenkinson MD (2011) Biology, genetics and imaging of glial cell tumours. Br J Radiol 84(2):S90–106. doi:10.1259/bjr/23430927
Fan GG, Deng QL, Wu ZH, Guo QY (2006) Usefulness of diffusion/perfusion-weighted MRI in patients with non-enhancing supratentorial brain gliomas: a valuable tool to predict tumour grading? Br J Radiol 79(944):652–658. doi:10.1259/bjr/25349497
Law M, Yang S, Babb JS, Knopp EA, Golfinos JG, Zagzag D, Johnson G (2004) Comparison of cerebral blood volume and vascular permeability from dynamic susceptibility contrast-enhanced perfusion MR imaging with glioma grade. AJNR Am J Neuroradiol 25(5):746–755
Cha S, Tihan T, Crawford F, Fischbein NJ, Chang S, Bollen A, Nelson SJ, Prados M, Berger MS, Dillon WP (2005) Differentiation of low-grade oligodendrogliomas from low-grade astrocytomas by using quantitative blood-volume measurements derived from dynamic susceptibility contrast-enhanced MR imaging. AJNR Am J Neuroradiol 26(2):266–273
Ostergaard L (2005) Principles of cerebral perfusion imaging by bolus tracking. J Magn Reson Imaging 22(6):710–717
Cha S, Knopp EA, Johnson G, Litt A, Glass J, Gruber ML, Lu S, Zagzag D (2000) Dynamic contrast-enhanced T2-weighted MR imaging of recurrent malignant gliomas treated with thalidomide and carboplatin. AJNR Am J Neuroradiol 21(5):881–890
Sourbron S, Ingrisch M, Siefert A, Reiser M, Herrmann K (2009) Quantification of cerebral blood flow, cerebral blood volume, and blood–brain-barrier leakage with DCE-MRI. Magn Reson Med: Off J Soc Magn Reson Med / Soc Magn Reson Med 62(1):205–217. doi:10.1002/mrm.22005
Larsson HB, Hansen AE, Berg HK, Rostrup E, Haraldseth O (2008) Dynamic contrast-enhanced quantitative perfusion measurement of the brain using T1-weighted MRI at 3 T. J Magn Reson Imaging 27(4):754–762. doi:10.1002/jmri.21328
Larsson HB, Courivaud F, Rostrup E, Hansen AE (2009) Measurement of brain perfusion, blood volume, and blood–brain barrier permeability, using dynamic contrast-enhanced T(1)-weighted MRI at 3 tesla. Magn Reson Med: Off J Soc Magn Reson Med / Soc Magn Reson Med 62(5):1270–1281. doi:10.1002/mrm.22136
Caseiras GB, Chheang S, Babb J, Rees JH, Pecerrelli N, Tozer DJ, Benton C, Zagzag D, Johnson G, Waldman AD, Jager HR, Law M (2010) Relative cerebral blood volume measurements of low-grade gliomas predict patient outcome in a multi-institution setting. Eur J Radiol 73(2):215–220. doi:10.1016/j.ejrad.2008.11.005
Knopp EA, Cha S, Johnson G, Mazumdar A, Golfinos JG, Zagzag D, Miller DC, Kelly PJ, Kricheff II (1999) Glial neoplasms: dynamic contrast-enhanced T2*-weighted MR imaging. Radiology 211(3):791–798
Law M, Yang S, Wang H, Babb JS, Johnson G, Cha S, Knopp EA, Zagzag D (2003) Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am J Neuroradiol 24(10):1989–1998
Hilario A, Ramos A, Perez-Nunez A, Salvador E, Millan JM, Lagares A, Sepulveda JM, Gonzalez-Leon P, Hernandez-Lain A, Ricoy JR (2012) The added value of apparent diffusion coefficient to cerebral blood volume in the preoperative grading of diffuse gliomas. AJNR Am J Neuroradiol 33(4):701–707. doi:10.3174/ajnr.A2846
Liu X, Tian W, Kolar B, Yeaney GA, Qiu X, Johnson MD, Ekholm S (2011) MR diffusion tensor and perfusion-weighted imaging in preoperative grading of supratentorial nonenhancing gliomas. Neuro-Oncology 13(4):447–455. doi:10.1093/neuonc/noq197
Khalid L, Carone M, Dumrongpisutikul N, Intrapiromkul J, Bonekamp D, Barker PB, Yousem DM (2012) Imaging characteristics of oligodendrogliomas that predict grade. AJNR Am J Neuroradiol 33(5):852–857. doi:10.3174/ajnr.A2895
Saito T, Yamasaki F, Kajiwara Y, Abe N, Akiyama Y, Kakuda T, Takeshima Y, Sugiyama K, Okada Y, Kurisu K (2012) Role of perfusion-weighted imaging at 3T in the histopathological differentiation between astrocytic and oligodendroglial tumors. Eur J Radiol 81(8):1863–1869. doi:10.1016/j.ejrad.2011.1804.1009, Epub 2011 May 1864
Berntsson SG, Falk A, Savitcheva I, Godau A, Zetterling M, Hesselager G, Alafuzoff I, Larsson E-M, Smits A (2013) Perfusion and diffusion MRI combined with 11C-methionine PET in the preoperative evaluation of suspected adult low-grade gliomas. J Neuro Oncol 114(2):241–249. doi:10.1007/s11060-013-1178-3
Tofts PS (1997) Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J Magn Reson Imaging 7(1):91–101
Boxerman JL, Schmainda KM, Weisskoff RM (2006) Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. AJNR Am J Neuroradiol 27(4):859–867
Ostergaard L (2005) Principles of cerebral perfusion imaging by bolus tracking. J Magn Reson Imaging 22(6):710–717. doi:10.1002/jmri.20460
Calamante F, Gadian DG, Connelly A (2003) Quantification of bolus-tracking MRI: improved characterization of the tissue residue function using Tikhonov regularization. Magn Reson Med: Off J Soc Magn Reson Med / Soc Magn Reson Med 50(6):1237–1247. doi:10.1002/mrm.10643
Young R, Babb J, Law M, Pollack E, Johnson G (2007) Comparison of region-of-interest analysis with three different histogram analysis methods in the determination of perfusion metrics in patients with brain gliomas. J Magn Reson Imaging 26(4):1053–1063. doi:10.1002/jmri.21064
Morita N, Wang S, Chawla S, Poptani H, Melhem ER (2010) Dynamic susceptibility contrast perfusion weighted imaging in grading of nonenhancing astrocytomas. J Magn Reson Imaging 32(4):803–808. doi:10.1002/jmri.22324
Lev MH, Ozsunar Y, Henson JW, Rasheed AA, Barest GD, Harsh GR, Fitzek MM, Chiocca EA, Rabinov JD, Csavoy AN, Rosen BR, Hochberg FH, Schaefer PW, Gonzalez RG (2004) Glial tumor grading and outcome prediction using dynamic spin-echo MR susceptibility mapping compared with conventional contrast-enhanced MR: confounding effect of elevated rCBV of oligodendrogliomas [corrected]. AJNR Am J Neuroradiol 25(2):214–221
Cha S, Yang L, Johnson G, Lai A, Chen MH, Tihan T, Wendland M, Dillon WP (2006) Comparison of microvascular permeability measurements, K(trans), determined with conventional steady-state T1-weighted and first-pass T2*-weighted MR imaging methods in gliomas and meningiomas. AJNR Am J Neuroradiol 27(2):409–417
Hakyemez B, Erdogan C, Ercan I, Ergin N, Uysal S, Atahan S (2005) High-grade and low-grade gliomas: differentiation by using perfusion MR imaging. Clin Radiol 60(4):493–502. doi:10.1016/j.crad.2004.09.009
Emblem KE, Nedregaard B, Nome T, Due-Tonnessen P, Hald JK, Scheie D, Borota OC, Cvancarova M, Bjornerud A (2008) Glioma grading by using histogram analysis of blood volume heterogeneity from MR-derived cerebral blood volume maps. Radiology 247(3):808–817. doi:10.1148/radiol.2473070571
Scott CB, Nelson JS, Farnan NC, Curran WJ Jr, Murray KJ, Fischbach AJ, Gaspar LE, Nelson DF (1995) Central pathology review in clinical trials for patients with malignant glioma. A report of Radiation Therapy Oncology Group 83–02. Cancer 76(2):307–313
Battaglini M, Jenkinson M, De Stefano N (2012) Evaluating and reducing the impact of white matter lesions on brain volume measurements. Hum Brain Mapp 33(9):2062–2071. doi:10.1002/hbm.21344
Larsen VA, Simonsen HJ, Law I, Larsson HB, Hansen AE (2013) Evaluation of dynamic contrast-enhanced T1-weighted perfusion MRI in the differentiation of tumor recurrence from radiation necrosis. Neuroradiology 55(3):361–369. doi:10.1007/s00234-012-1127-4
Ethical standards and patient consent
We declare that all human studies have been approved by the Uppsala Ethics Committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that all patients gave informed consent prior to inclusion in this study.
Acknowledgments
The authors would like to thank Lars Berglund, Statistician, Uppsala Clinical Research Center, Sweden, Monika Gelotte, Research Assistant, Uppsala University, Sweden, and Håkan Petterson, IT Manager, Uppsala University, Sweden.
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Elna-Marie Larsson and Anja Smits share senior authorship.
Rights and permissions
About this article
Cite this article
Falk, A., Fahlström, M., Rostrup, E. et al. Discrimination between glioma grades II and III in suspected low-grade gliomas using dynamic contrast-enhanced and dynamic susceptibility contrast perfusion MR imaging: a histogram analysis approach. Neuroradiology 56, 1031–1038 (2014). https://doi.org/10.1007/s00234-014-1426-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-014-1426-z