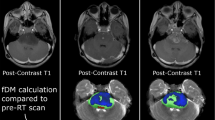
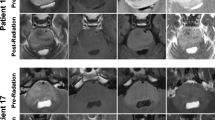
Abstract
Introduction
Based on clinical observations, we hypothesized that in infiltrative high-grade brainstem neoplasms, such as diffuse intrinsic pontine glioma (DIPG), longitudinal metabolic evaluation of the tumor by magnetic resonance spectroscopy (MRS) may be more accurate than volumetric data for monitoring the tumor’s biological evolution during standard treatment.
Methods
We evaluated longitudinal MRS data and corresponding tumor volumes of 31 children with DIPG. We statistically analyzed correlations between tumor volume and ratios of Cho/NAA, Cho/Cr, and NAA/Cr at key time points during the course of the disease through the end of the progression-free survival period.
Results
By the end of RT, tumor volume had significantly decreased from the baseline (P < .0001) and remained decreased through the last available follow-up magnetic resonance imaging study (P = .007632). However, the metabolic profile of the tumor tissue (Cho/Cr, NAA/Cr, and Cho/NAA ratios) did not change significantly over time.
Conclusion
Our data show that longitudinal tumor volume and metabolic profile changes are dissociated in patients with DIPG during progression-free survival. Volume changes, therefore, may not accurately reflect treatment-related changes in tumor burden. This study adds to the existing body of evidence that the value of conventional MRI metrics, including volumetric data, needs to be reevaluated critically and, in infiltrative tumors in particular, may not be useful as study end-points in clinical trials. We submit that advanced quantitative MRI data, including robust, MRS-based metabolic ratios and diffusion and perfusion metrics, may be better surrogate markers of key end-points in clinical trials.


Similar content being viewed by others
References
CBTRUS (2010) CBTRUS. Statistical Report: Primary brain and central nervous system tumors diagnosed in the United States in 2004–2006
Albright AL, Price RA, Guthkelch AN (1983) Brain stem gliomas of children. A clinicopathological study. Cancer 52:2313–2319
Epstein F, McCleary EL (1986) Intrinsic brain-stem tumors of childhood: surgical indications. J Neurosurg 64:11–15. doi:10.3171/jns.1986.64.1.0011
Kaplan AM, Albright AL, Zimmerman RA et al (1996) Brainstem gliomas in children. A Children’s Cancer Group review of 119 cases. Pediatr Neurosurg 24:185–192
Thakur SB, Karimi S, Dunkel IJ et al (2006) Longitudinal MR spectroscopic imaging of pediatric diffuse pontine tumors to assess tumor aggression and progression. AJNR Am J Neuroradiol 27:806–809
Hargrave D, Chuang N, Bouffet E (2008) Conventional MRI cannot predict survival in childhood diffuse intrinsic pontine glioma. J Neurooncol 86:313–319. doi:10.1007/s11060-007-9473-5
Liu AK, Brandon J, Foreman NK, Fenton LZ (2009) Conventional MRI at presentation does not predict clinical response to radiation therapy in children with diffuse pontine glioma. Pediatr Radiol 39:1317–1320. doi:10.1007/s00247-009-1368-5
Hargrave D, Bartels U, Bouffet E (2006) Diffuse brainstem glioma in children: critical review of clinical trials. Lancet Oncol 7:241–248. doi:10.1016/S1470-2045(06)70615-5
Hipp SJ, Steffen-Smith E, Hammoud D et al (2011) Predicting outcome of children with diffuse intrinsic pontine gliomas using multiparametric imaging. Neurooncol 13:904–909. doi:10.1093/neuonc/nor076
Panigrahy A, Nelson MD, Finlay JL et al (2008) Metabolism of diffuse intrinsic brainstem gliomas in children. Neurooncol 10:32–44. doi:10.1215/15228517-2007-042
Steffen-Smith EA, Shih JH, Hipp SJ et al (2011) Proton magnetic resonance spectroscopy predicts survival in children with diffuse intrinsic pontine glioma. J Neurooncol 105:365–373. doi:10.1007/s11060-011-0601-x
Broniscer A, Baker JN, Tagen M et al (2010) Phase I study of vandetanib during and after radiotherapy in children with diffuse intrinsic pontine glioma. J Clin Oncol 28:4762–4768. doi:10.1200/JCO.2010.30.3545
Yamasaki F, Kurisu K, Kajiwara Y et al (2011) Magnetic resonance spectroscopic detection of lactate is predictive of a poor prognosis in patients with diffuse intrinsic pontine glioma. Neurooncol 13:791–801. doi:10.1093/neuonc/nor038
Burger PC, Mahley MS, Dudka L, Vogel FS (1979) The morphologic effects of radiation administered therapeutically for intracranial gliomas: a postmortem study of 25 cases. Cancer 44:1256–1272
Dietrich J, Rao K, Pastorino S, Kesari S (2011) Corticosteroids in brain cancer patients: benefits and pitfalls. Expert Rev Clin Pharmacol 4:233–242. doi:10.1586/ecp.11.1
Machein MR, Plate KH (2000) VEGF in brain tumors. J Neurooncol 50:109–120
Hedley-Whyte ET, Hsu DW (1986) Effect of dexamethasone on blood–brain barrier in the normal mouse. Ann Neurol 19:373–377. doi:10.1002/ana.410190411
Heiss JD, Papavassiliou E, Merrill MJ et al (1996) Mechanism of dexamethasone suppression of brain tumor-associated vascular permeability in rats. Involvement of the glucocorticoid receptor and vascular permeability factor. J Clin Invest 98:1400–1408. doi:10.1172/JCI118927
Papadopoulos MC, Saadoun S, Binder DK et al (2004) Molecular mechanisms of brain tumor edema. Neuroscience 129:1011–1020. doi:10.1016/j.neuroscience.2004.05.044
Sedlacik J, Winchell A, Kocak M et al (2013) MR imaging assessment of tumor perfusion and 3D segmented volume at baseline, during treatment, and at tumor progression in children with newly diagnosed diffuse intrinsic pontine glioma. AJNR Am J Neuroradiol 34:1450–1455. doi:10.3174/ajnr.A3421
Poussaint TY, Kocak M, Vajapeyam S et al (2011) MRI as a central component of clinical trials analysis in brainstem glioma: a report from the Pediatric Brain Tumor Consortium (PBTC). Neuro-Oncol 13:417–427. doi:10.1093/neuonc/noq200
Stadlbauer A, Moser E, Gruber S et al (2004) Improved delineation of brain tumors: an automated method for segmentation based on pathologic changes of 1H-MRSI metabolites in gliomas. NeuroImage 23:454–461. doi:10.1016/j.neuroimage.2004.06.022
Macdonald DR, Cascino TL, Schold SC, Cairncross JG (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280
Wen PY, Macdonald DR, Reardon DA et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972. doi:10.1200/JCO.2009.26.3541
Warren KE, Poussaint TY, Vezina G et al (2013) Challenges with defining response to antitumor agents in pediatric neuro-oncology: a report from the response assessment in pediatric neuro-oncology (RAPNO) working group. Pediatr Blood Cancer 60:1397–1401. doi:10.1002/pbc.24562
Kelly PJ, Daumas-Duport C, Scheithauer BW et al (1987) Stereotactic histologic correlations of computed tomography- and magnetic resonance imaging-defined abnormalities in patients with glial neoplasms. Mayo Clin Proc 62:450–459
Lu S, Ahn D, Johnson G et al (2004) Diffusion-tensor MR imaging of intracranial neoplasia and associated peritumoral edema: introduction of the tumor infiltration index. Radiology 232:221–228. doi:10.1148/radiol.2321030653
Oh J, Cha S, Aiken AH et al (2005) Quantitative apparent diffusion coefficients and T2 relaxation times in characterizing contrast enhancing brain tumors and regions of peritumoral edema. J Magn Reson Imaging JMRI 21:701–708. doi:10.1002/jmri.20335
Cohen BA, Knopp EA, Rusinek H et al (2005) Assessing global invasion of newly diagnosed glial tumors with whole-brain proton MR spectroscopy. AJNR Am J Neuroradiol 26:2170–2177
Steffen-Smith EA, Venzon DJ, Bent RS et al (2012) Single- and multivoxel proton spectroscopy in pediatric patients with diffuse intrinsic pontine glioma. Int J Radiat Oncol Biol Phys 84:774–779. doi:10.1016/j.ijrobp.2012.01.032
Law M, Yang S, Wang H et al (2003) Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am J Neuroradiol 24:1989–1998
Laprie A, Pirzkall A, Haas-Kogan DA et al (2005) Longitudinal multivoxel MR spectroscopy study of pediatric diffuse brainstem gliomas treated with radiotherapy. Int J Radiat Oncol Biol Phys 62:20–31. doi:10.1016/j.ijrobp.2004.09.027
Poussaint TY, Vajapeyam S, Ricci KI, et al. (2015) Apparent diffusion coefficient histogram metrics correlate with survival in diffuse intrinsic pontine glioma: a report from the Pediatric Brain Tumor Consortium. Neuro Oncol
Acknowledgments
This work was supported in part by Grant P30 CA021765 from the National Cancer Institute and by the American Lebanese Syrian Associated Charities (ALSAC). The authors thank Dr. Cherise Guess of the Department of Scientific Editing at St. Jude Children’s Research Hospital for editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
We declare that all human studies have been approved by the local ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that all patients gave informed consent prior to inclusion in this study.
Conflict of interest
AB received partial financial support from AstraZeneca to conduct this clinical trial.
Rights and permissions
About this article
Cite this article
Löbel, U., Hwang, S., Edwards, A. et al. Discrepant longitudinal volumetric and metabolic evolution of diffuse intrinsic Pontine gliomas during treatment: implications for current response assessment strategies. Neuroradiology 58, 1027–1034 (2016). https://doi.org/10.1007/s00234-016-1724-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-016-1724-8