Abstract
Purpose
The discussed topic about gadolinium-based contrast agents (GBCA) safety has recently been revived due to the evidence of hyperintensities observed in the dentate nucleus (DN) and globus pallidus (GP) in the brain of patients with normal kidney function. Several preclinical studies have been conducted to understanding how the use of GBCAs can promote the gadolinium deposition in the brain. Here, we evaluate the impact of chronic cerebral hypoperfusion on gadolinium presence.
Methods
T1 hyperintensities and BBB integrity were evaluated by MRI in chronically hypoperfused and healthy rats injected with either gadodiamide or hypertonic saline. Additionally, the assessment of glucose metabolism by PET imaging and the gadolinium content by ICP-MS was performed after the last MR scan.
Results
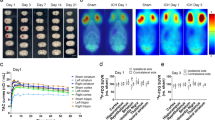
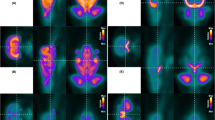
Chronically hypoperfused rats displayed a greater MRI T1w signal in the DCN and hippocampus compared to Sham-operated animals, suggesting gadolinium accumulation. Dynamic contrast-enhanced (DCE) MRI assessment of BBB permeability revealed loss of integrity (high Ktrans) after rat injury in the dentate nuclei and hippocampus. Ex vivo tissue analysis showed greater gadolinium retention in the cerebellum and subcortical regions, supporting the imaging finding. FDG-PET imaging of the cerebellum did not reveal abnormal uptake in the DCN after chronic cerebral hypoperfusion.
Conclusion
Higher signal intensity followed by higher Gd concentration observed in DCN and hippocampus of animals subjected to cerebral injury can be associated with an increase in BBB permeability due to the applied vascular dementia animal model. Nonetheless, no glucose metabolism abnormalities were detected in chronically hypoperfused cerebellum.






Similar content being viewed by others
References
McDonald RJ, McDonald JS, Kallmes DF, Jentoft ME, Murray DL, Thielen KR, Williamson EE, Eckel LJ (2015) Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 275(3):772–782. https://doi.org/10.1148/radiol.15150025
Errante Y, Cirimele V, Mallio CA, Di Lazzaro V, Zobel BB, Quattrocchi CC (2014) Progressive increase of T1 signal intensity of the dentate nucleus on unenhanced magnetic resonance images is associated with cumulative doses of intravenously administered gadodiamide in patients with normal renal function, suggesting dechelation. Investig Radiol 49(10):685–690
Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D (2014) High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 270(3):834–841. https://doi.org/10.1148/radiol.13131669
Quattrocchi CC, Mallio CA, Errante Y, Cirimele V, Carideo L, Ax A, Zobel BB (2015) Gadodiamide and dentate nucleus T1 hyperintensity in patients with meningioma evaluated by multiple follow-up contrast-enhanced magnetic resonance examinations with no systemic interval therapy. Investig Radiol 50(7):470–472. https://doi.org/10.1097/RLI.0000000000000154
Kalaria RN, Akinyemi R, Ihara M (2012) Does vascular pathology contribute to Alzheimer changes? J Neurol Sci 322(1–2):141–147. https://doi.org/10.1016/j.jns.2012.07.032
Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, Harrington MG, Chui HC, Law M, Zlokovic BV (2015) Blood-brain barrier breakdown in the aging human hippocampus. Neuron 85(2):296–302. https://doi.org/10.1016/j.neuron.2014.12.032
Jost G, Lenhard DC, Sieber MA, Lohrke J, Frenzel T, Pietsch H (2016) Signal increase on unenhanced T1-weighted images in the rat brain after repeated, extended doses of gadolinium-based contrast agents Comparison of Linear and Macrocyclic Agents. Invest Radiol 51(2):83–89. https://doi.org/10.1097/RLI.0000000000000242
Gianolio E, Bardini P, Arena F, Stefania R, Di Gregorio E, Iani R, Aime S (2017) Gadolinium retention in the rat brain: assessment of the amounts of insoluble gadolinium-containing species and intact gadolinium complexes after repeated administration of gadolinium-based contrast agents. Radiology 162857. https://doi.org/10.1148/radiol.2017162857
Robert P, Fingerhut S, Factor C, Vives V, Letien J, Sperling M, Rasschaert M, Santus R, Ballet S, Idee JM, Corot C, Karst U (2018) One-year retention of gadolinium in the brain: comparison of gadodiamide and gadoterate meglumine in a rodent model. Radiology 172746:424–433. https://doi.org/10.1148/radiol.2018172746
Robert P, Frenzel T, Factor C, Jost G, Rasschaert M, Schuetz G, Fretellier N, Boyken J, Idee JM, Pietsch H (2018) Methodological aspects for preclinical evaluation of gadolinium presence in brain tissue: critical appraisal and suggestions for harmonization-a joint initiative. Investig Radiol 53:499–517. https://doi.org/10.1097/RLI.0000000000000467
Farkas E, Luiten PG, Bari F (2007) Permanent, bilateral common carotid artery occlusion in the rat: a model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res Rev 54(1):162–180. https://doi.org/10.1016/j.brainresrev.2007.01.003
Wang ZQ, Fan J, Wang J, Li YX, Duan D, Du G, Wang QS (2016) Chronic cerebral hypoperfusion induces long-lasting cognitive deficits accompanied by long-term hippocampal silent synapses increase in rats. Behav Brain Res 301:243–252. https://doi.org/10.1016/j.bbr.2015.12.047
Soria G, Tudela R, Marquez-Martin A, Camon L, Batalle D, Munoz-Moreno E, Eixarch E, Puig J, Pedraza S, Vila E, Prats-Galino A, Planas AM (2013) The ins and outs of the BCCAo model for chronic hypoperfusion: a multimodal and longitudinal MRI approach. PLoS One 8(9):e74631. https://doi.org/10.1371/journal.pone.0074631
Guidance for industry. Estimating the maximum safe. Starting dose in initial clinical trials for therapeutics in adult healthy volunteers (2005) U.S. Department of Health and Human Services. Food and Drug Administration. Center for Drug Evaluation and Research (CDER)
Faraco G, Blasi F, Min W, Wang ZQ, Moroni F, Chiarugi A (2010) Brain ischemic preconditioning does not require PARP-1. Stroke 41(1):181–183. https://doi.org/10.1161/STROKEAHA.109.567826
Choki JI, Yamaguchi T, Takeya Y, Morotomi Y, Omae T (1977) Effect of carotid artery ligation on regional cerebral blood flow in normotensive and spontaneously hypertensive rats. Stroke 8(3):374–379
Brookes JA, Redpath TW, Gilbert FJ, Murray AD, Staff RT (1999) Accuracy of T1 measurement in dynamic contrast-enhanced breast MRI using two- and three-dimensional variable flip angle fast low-angle shot. J Magn Reson Imaging 9(2):163–171
Paxinos GWC (ed) (2008) The rat brain in stereotaxic coordinates, 6th edn. San Diego, CA
Blasi F, Oliveira BL, Rietz TA, Rotile NJ, Day H, Naha PC, Cormode DP, Izquierdo-Garcia D, Catana C, Caravan P (2015) Radiation dosimetry of the fibrin-binding probe (6)(4)Cu-FBP8 and its feasibility for PET imaging of deep vein thrombosis and pulmonary embolism in rats. J Nucl Med 56(7):1088–1093. https://doi.org/10.2967/jnumed.115.157982
Loening AM, Gambhir SS (2003) AMIDE: a free software tool for multimodality medical image analysis. Mol Imaging 2(3):131–137
Longo DL, Bartoli A, Consolino L, Bardini P, Arena F, Schwaiger M, Aime S (2016) In vivo imaging of tumor metabolism and acidosis by combining PET and MRI-CEST pH imaging. Cancer Res 76(22):6463–6470. https://doi.org/10.1158/0008-5472.CAN-16-0825
Tsuchiya M, Sako K, Yura S, Yonemasu Y (1992) Cerebral blood flow and histopathological changes following permanent bilateral carotid artery ligation in Wistar rats. Exp Brain Res 89(1):87–92
Larsson HB, Courivaud F, Rostrup E, Hansen AE (2009) Measurement of brain perfusion, blood volume, and blood-brain barrier permeability, using dynamic contrast-enhanced T(1)-weighted MRI at 3 tesla. Magn Reson Med 62(5):1270–1281. https://doi.org/10.1002/mrm.22136
Kanda T, Fukusato T, Matsuda M, Toyoda K, Oba H, Kotoku J, Haruyama T, Kitajima K, Furui S (2015) Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 276(1):228–232. https://doi.org/10.1148/radiol.2015142690
Radbruch A, Weberling LD, Kieslich PJ, Eidel O, Burth S, Kickingereder P, Heiland S, Wick W, Schlemmer HP, Bendszus M (2015) Gadolinium retention in the dentate nucleus and globus pallidus is dependent on the class of contrast agent. Radiology 275(3):783–791. https://doi.org/10.1148/radiol.2015150337
Robert P, Violas X, Grand S, Lehericy S, Idee JM, Ballet S, Corot C (2016) Linear gadolinium-based contrast agents are associated with brain gadolinium retention in healthy rats. Investig Radiol 51(2):73–82. https://doi.org/10.1097/RLI.0000000000000241
Robert P, Lehericy S, Grand S, Violas X, Fretellier N, Idee JM, Ballet S, Corot C (2015) T1-weighted hypersignal in the deep cerebellar nuclei after repeated administrations of gadolinium-based contrast agents in healthy rats: difference between linear and macrocyclic agents. Investig Radiol 50(8):473–480. https://doi.org/10.1097/RLI.0000000000000181
Roberts DR, Lindhorst SM, Welsh CT, Maravilla KR, Herring MN, Braun KA, Thiers BH, Davis WC (2016) High levels of gadolinium deposition in the skin of a patient with normal renal function. Investig Radiol 51(5):280–289. https://doi.org/10.1097/RLI.0000000000000266
Sharma HS (2003) Blood-spinal cord and brain barriers in health and disease. 1st Edition edn.,
Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, Wu Z, Holtzman DM, Betsholtz C, Armulik A, Sallstrom J, Berk BC, Zlokovic BV (2012) Apolipoprotein E controls cerebrovascular integrity via cyclophilin a. Nature 485(7399):512–516. https://doi.org/10.1038/nature11087
Li W, Watts L, Long J, Zhou W, Shen Q, Jiang Z, Li Y, Duong TQ (2016) Spatiotemporal changes in blood-brain barrier permeability, cerebral blood flow, T2 and diffusion following mild traumatic brain injury. Brain Res 1646:53–61. https://doi.org/10.1016/j.brainres.2016.05.036
Reimer MM, McQueen J, Searcy L, Scullion G, Zonta B, Desmazieres A, Holland PR, Smith J, Gliddon C, Wood ER, Herzyk P, Brophy PJ, McCulloch J, Horsburgh K (2011) Rapid disruption of axon-glial integrity in response to mild cerebral hypoperfusion. J Neurosci 31(49):18185–18194. https://doi.org/10.1523/JNEUROSCI.4936-11.2011
Aksoy D, Bammer R, Mlynash M, Venkatasubramanian C, Eyngorn I, Snider RW, Gupta SN, Narayana R, Fischbein N, Wijman CA (2013) Magnetic resonance imaging profile of blood-brain barrier injury in patients with acute intracerebral hemorrhage. J Am Heart Assoc 2(3):e000161. https://doi.org/10.1161/JAHA.113.000161
Montagne A, Toga AW, Zlokovic BV (2016) Blood-brain barrier permeability and gadolinium: benefits and potential pitfalls in research. JAMA neurology 73(1):13–14. https://doi.org/10.1001/jamaneurol.2015.2960
Mosconi L, Mistur R, Switalski R, Tsui WH, Glodzik L, Li Y, Pirraglia E, De Santi S, Reisberg B, Wisniewski T, de Leon MJ (2009) FDG-PET changes in brain glucose metabolism from normal cognition to pathologically verified Alzheimer's disease. Eur J Nucl Med Mol Imaging 36(5):811–822. https://doi.org/10.1007/s00259-008-1039-z
Alavi JB, Alavi A, Chawluk J, Kushner M, Powe J, Hickey W, Reivich M (1988) Positron emission tomography in patients with glioma. A predictor of prognosis. Cancer 62(6):1074–1078
Bauer K, Lathrum A, Raslan O, Kelly PV, Zhou Y, Hewing D, Botkin C, Turner JA, Osman M (2017) Do gadolinium-based contrast agents affect (18)F-FDG PET/CT uptake in the dentate nucleus and the globus pallidus? A pilot study. J Nucl Med Technol 45(1):30–33. https://doi.org/10.2967/jnmt.116.180844
Naganawa S (2017) Effect of gadolinium deposition on (18)F-FDG PET/CT of dentate nucleus and globus pallidus. J Nucl Med Technol 45(2):173–17173. https://doi.org/10.2967/jnmt.116.187591
Kanal E, Tweedle MF (2015) Residual or retained gadolinium: practical implications for radiologists and our patients. Radiology 275(3):630–634. https://doi.org/10.1148/radiol.2015150805
Gulani V, Calamante F, Shellock FG, Kanal E, Reeder SB, International Society for Magnetic Resonance in M (2017) Gadolinium deposition in the brain: summary of evidence and recommendations. Lancet Neurol 16(7):564–570. https://doi.org/10.1016/S1474-4422(17)30158-8
Kanal E, Maravilla K, Rowley HA (2014) Gadolinium contrast agents for CNS imaging: current concepts and clinical evidence. AJNR Am J Neuroradiol 35(12):2215–2226. https://doi.org/10.3174/ajnr.A3917
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this study.
Conflict of interest
SA consults for Bracco Imaging (Milan, Italy).
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed (L.D. 26/2014; Directives 2010/63/EU). Every effort was made to minimize the number of animals used and their suffering.
Informed consent
Statement of informed consent was not applicable since the manuscript does not contain any patient data.
Rights and permissions
About this article
Cite this article
Arena, F., Bardini, P., Blasi, F. et al. Gadolinium presence, MRI hyperintensities, and glucose uptake in the hypoperfused rat brain after repeated administrations of gadodiamide. Neuroradiology 61, 163–173 (2019). https://doi.org/10.1007/s00234-018-2120-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-018-2120-3