ABSTRACT
Purpose
Impaired olfactory function is one of the main features of Parkinson’s disease. However, how peripheral olfactory structures are involved remains unclear. Using diffusion tensor imaging fiber tracking, we investigated for MRI microstructural changes in the parkinsonian peripheral olfactory system and particularly the olfactory tract, in order to seek a better understanding of the structural alternations underlying hyposmia in Parkinson’s disease.
Methods
All patients were assessed utilizing by the Italian Olfactory Identification Test for olfactory function and the Unified Parkinson’s Disease Rating Scale-III part as well as Hoehn and Yahr rating scale for motor disability. Imaging was performed on a 3 T Clinical MR scanner. MRI data pre-processing was carried out by DTIPrep, diffusion tensor imaging reconstruction, and fiber tracking using Diffusion Toolkit and tractography analysis by TrackVis. The following parameters were used for groupwise comparison: fractional anisotropy, mean diffusivity, radial diffusivity, axial diffusivity, and tract volume.
Results
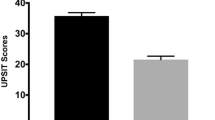
Overall 23 patients with Parkinson’s disease (mean age 63.6 ± 9.3 years, UPDRS-III 24.5 ± 12.3, H&Y 1.9 ± 0.5) and 18 controls (mean age 56.3 ± 13.7 years) were recruited. All patients had been diagnosed hyposmic. Diffusion tensor imaging analysis of the olfactory tract showed significant fractional anisotropy, and tract volume decreases for the Parkinson’s disease group compared with controls (P < 0.05). Fractional anisotropy and age, in the control group, were significant for multiple correlations (r = − 0.36, P < 0.05, Spearman’s rank correlation).
Conclusions
Fiber tracking diffusion tensor imaging analysis of olfactory tract was feasible, and it could be helpful for characterizing hyposmia in Parkinson’s disease.



Similar content being viewed by others
References
Chaudhuri KR, Healy DG, Schapira AH (2006) Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol 5:235–245
Hawkes CH, Del Tredici K, Braak H (2007) Parkinson’s disease: a dual-hit hypothesis. Neuropathol Appl Neurobiol 33:599–614
Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211
Hawkes CH (2008) The prodromal phase of sporadic Parkinson’s disease: does it exist and if so how long is it? Mov Disord 23:1799–1807
Fullard ME, Morley JF, Duda JE (2017) Olfactory dysfunction as an early biomarker in Parkinson’s disease. Neurosci Bull 33:515–525
Postuma RB, Berg D (2016) Advances in markers of prodromal Parkinson disease. Nat Rev Neurol 12:622–634
Hummel T, Witt M, Reichmann H, Welge-Luessen A, Haehner A (2009) Immunohistochemical, volumetric, and functional neuroimaging studies in patients with idiopathic Parkinson’s disease. J Neurol Sci 289:119–122
Silveira-Moriyama L, Holton JL, Kingsbury A, Ayling H, Petrie A, Sterlacci W, Poewe W, Maier H, Lees AJ, Revesz T (2009) Regional differences in the severity of Lewy body pathology across the olfactory cortex. Neurosci Lett 453:77–80
Del Tredici K, Ru BU, De Vos RA, Bohl JR, Braak H (2002) Where does parkinson disease pathology begin in the brain? J Neuropathol Exp Neurol 61:413–426
Politis M, Wu K, Molloy SG, Bain P, Chaudhuri KR, Piccini P (2010) Parkinson’s disease symptoms: the patient’s perspective. Mov Disord 25:1646–1651
Brooks DJ, Tambasco N (2016) Imaging synucleinopathies. Mov Disord 31:814–829
Su M, Wang S, Fang W, Zhu Y, Li R, Sheng K, Zou D, Han Y, Wang X, Cheng O (2015) Alterations in the limbic/paralimbic cortices of Parkinson’s disease patients with hyposmia under resting-state functional MRI by regional homogeneity and functional connectivity analysis. Parkinsonism Relat Disord 21(7):698–703
Scherfler C, Schocke MF, Seppi K, Esterhammer R, Brenneis C, Jaschke W, Wenning GK, Poewe W (2006) Voxel-wise analysis of diffusion weighted imaging reveals disruption of the olfactory tract in Parkinson’s disease. Brain 129:538–542
Zhang K, Yu C, Zhang Y, Wu X, Zhu C, Chan P, Li K (2011) Voxel-based analysis of diffusion tensor indices in the brain in patients with Parkinson’s disease. Eur J Radiol 77:269–273
Ibarretxe-Bilbao N, Junque C, Marti MJ, Valldeoriola F, Vendrell P, Bargallo N, Zarei M, Tolosa E (2010) Olfactory impairment in Parkinson’s disease and white matter abnormalities in central olfactory areas: a voxel-based diffusion tensor imaging study. Mov Disord 25:1888–1894
Basser PJ, Mattiello J, LeBihan D (1994) MR diffusion tensor spectroscopy and imaging. Biophys J 66:259–267
Alexander AL, Lee JE, Lazar M, Field AS (2007) Diffusion tensor imaging of the brain. Neurotherapeutics 4:316–329
Abhinav K, Yeh FC, Pathak S, Suski V, Lacomis D, Friedlander RM, Fernandez-Miranda JC (2014) Advanced diffusion MRI fiber tracking in neurosurgical and neurodegenerative disorders and neuroanatomical studies: a review. Biochim Biophys Acta 1842:2286–2297
Schulte T, Sullivan EV, Müller-Oehring EM, Adalsteinsson E, Pfefferbaum A (2005) Corpus callosal microstructural integrity influences interhemispheric processing: a diffusion tensor imaging study. Cereb Cortex 15:1384–1392
Skorpil M, Rolheiser T, Robertson H, Sundin A, Svenningsson P (2011) Diffusion tensor fiber tractography of the olfactory tract. Magn Reson Imaging 29:289–292
Daniel SE, Lees AJ (1993) Parkinson’s Disease Society Brain Bank, London: overview and research. J Neural Transm Suppl 39:165–172
Maremmani C, Rossi G, Tambasco N, Fattori B, Pieroni A, Ramat S, Napolitano A, Vanni P, Serra P, Piersanti P, Zanetti M, Coltelli M, Orsini M, Marconi R, Purcaro C, Rossi A, Calabresi P, Meco G (2012) The validity and reliability of the Italian Olfactory Identification Test (IOIT) in healthy subjects and in Parkinson’s disease patients. Parkinsonism Relat Disord 18:788–793
Rl D, Stern MB, Pfeiffer C, Gollomp SM, Hurtig HI (1992) Bilateral olfactory dysfunction in early stage treated and untreated idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry 55:128–142 20
Scherfler C, Esterhammer R, Nocker M, Mahlknecht P, Stockner H, Warwitz B, Spielberger S, Pinter B, Donnemiller E, Decristoforo C, Virgolini I, Schocke M, Poewe W, Seppi K (2013) Correlation of dopaminergic terminal dysfunction and microstructural abnormalities of the basal ganglia and the olfactory tract in Parkinson’s disease. Brain 136:3028–3037
Oguz I, Farzinfar M, Matsui J, Budin F, Liu Z, Gerig G, Johnson HJ, Styner M (2014) DTIPrep: quality control of diffusion-weighted images. Front Neuroinform 8:4
Wang R, Benner T, Sorensen AG et al (2007) Diffusion toolkit: a software package for diffusion imaging data processing and tractography. Proc Intl Soc Mag Reson Med 15:3720
Atkinson-Clement C, Pinto S, Eusebio A, Coulon O (2017) Diffusion tensor imaging in Parkinson’s disease: review and meta-analysis. NeuroImage Clin 16:98–110
Rolheiser TM, Fulton HG, Good KP, Fisk JD, McKelvey JR, Scherfler C, Khan NM, Leslie RA, Robertson HA (2011) Diffusion tensor imaging and olfactory identification testing in early-stage Parkinson’s disease. J Neurol 258:1254–1260
Chen NK, Chou YH, Sundman M, Hickey P, Kasoff WS, Bernstein A, Trouard TP, Lin T, Rapcsak SZ, Sherman SJ, Weingarten CP (2018) Alteration of diffusion-tensor magnetic resonance imaging measures in brain regions involved in early stages of Parkinson’s disease. Brain Connect 8:343–349
Nigro S, Riccelli R, Passamonti L, Arabia G, Morelli M, Nisticò R, Novellino F, Salsone M, Barbagallo G, Quattrone A (2016) Characterizing structural neural networks in de novo Parkinson disease patients using diffusion tensor imaging: altered structural brain network in drug-Naïve PD. Hum Brain Mapp 37:4500–4510
Dando SJ, Mackay-Sim A, Norton R, Currie BJ, St John JA, Ekberg JA, Batzloff M, Ulett GC, Beacham IR (2014) Pathogens penetrating the central nervous system: infection pathways and the cellular and molecular mechanisms of invasion. Clin Microbiol Rev 27:691–726
Doty RL (2008) The olfactory vector hypothesis of neurodegenerative disease: is it viable? Ann Neurol 63:7–15
Rey NL, Wesson DW, Brundin P (2018) The olfactory bulb as the entry site for prion-like propagation in neurodegenerative diseases. Neurobiol Dis 109:226–248
Aguzzi A, Baumann F, Bremer J (2008) The Prion’s elusive reason for being. Annu Rev Neurosci 31:439–477
Acknowledgments
The Movement Disorders Center of the University of Perugia was supported by a grant from the New York University School of Medicine and the Marlene and Paolo Fresco Institute for Parkinson’s and Movement Disorders, which was made possible with support from Marlene and Paolo Fresco.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
All authors have approved the final article.
Corresponding author
Ethics declarations
Conflict of interest
NT received a travel grant from Abbvie. PaC received research support from Bayer Schering, Biogen-Dompé, Boehringer Ingelheim, Eisai, Lundbeck, Merck-Serono, Novartis, Sanofi-Aventis, Sigma-Tau, and UCB Pharma. PN, AC, PE, SS, FPP, GC, PC, MF, RT, GG, VS, and LP declare that there are no disclosures to report.
Ethical approval
The study was reviewed and approved by the local ethic committee.
Informed consent
After a full explanation of the study, written informed consent was obtained from all participants according to the Declaration of Helsinki.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nigro, P., Chiappiniello, A., Simoni, S. et al. Changes of olfactory tract in Parkinson’s disease: a DTI tractography study. Neuroradiology 63, 235–242 (2021). https://doi.org/10.1007/s00234-020-02551-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-020-02551-4