Abstract
We evaluated the presence of pulmonary artery diverticulum in patients with Williams syndrome in comparison with other conditions causing peripheral pulmonary artery stenosis (PPS). Angiographic characteristics of patients with a definitive diagnosis of Williams syndrome, by fluorescence in situ hybridization, between 1990 and 2008 were reviewed. These data were compared with those diagnosed with those for patients with PPS without Williams syndrome. Differentiating morphological features on angiography were compared between the groups, along with demographic and echocardiographic data. Twelve patients with a chromosomal diagnosis of Williams syndrome who underwent cardiac catheterization were identified. Seven were male. Eleven patients (91%) had supravalvar aortic stenosis and nine (81%) had PPS. Pulmonary valve stenosis was seen in two patients. Eight patients who were negative for Williams syndrome and had PPS were identified during the same period. Two had Alagille syndrome and one had Noonan syndrome. Mean age at catheterization was 5 years in the Williams group versus 8 years in the non-Williams group. Pulmonary artery diverticulum involving the main pulmonary artery was documented in all patients with Williams syndrome, while none of the patients in the other group had it. It originated at the bifurcation of the pulmonary artery in all. In conclusion, the angiographic appearance of a diverticulum as an extension of the main pulmonary artery is a consistent finding in patients with Williams syndrome. Compared to the classically described findings of supravalvar aortic stenosis or PPS, pulmonary artery diverticulum can be considered as a pathognomonic feature of Williams syndrome.
Similar content being viewed by others
Williams syndrome (WS) is a multisystem disorder affecting approximately 1 in 20,000 live births. Sixty to 80% of patients have associated congenital cardiovascular malformations [3–5]. Supravalvar aortic stenosis (SVAS) is the most common cardiac defect and peripheral pulmonary artery stenosis (PPS) is the second most commonly identified lesion [14]. The PPS is often multiple and bilateral and can be associated with hypoplasia of the pulmonary arterial bed [7]. Pulmonary artery diverticulum (an outpouching of the main pulmonary artery extending cranially between the origins of the left and right pulmonary arteries) has not been described as an associated finding in patients with WS.
This finding was observed in our patient population with WS. After noticing that, in a series of patients with WS, a retrospective analysis was done, and the main objective of this study was to describe the presence of pulmonary artery diverticulum as a consistent feature of WS on angiography.
Methods
Patients admitted between 1990 and 2008 with a diagnosis of PPS or WS who underwent cardiac catheterization formed the cohort of this retrospective study. They were divided into two groups. WS confirmed by genetic testing using fluorescent in situ hybridization technique formed the first group. These data were compared with those for a second group diagnosed with PPS who were negative for the genetic marker for WS.
Cardiac evaluation included physical examination, electrocardiography, and echocardiography. Only patients who underwent cardiac catheterization and had cineangiograms available for review were included in the cohort.
All catheter procedures were done under general anesthesia using the femoral approach. Anteroposterior and lateral images were obtained for right ventricle angiogram. Pulmonary artery diverticulum was defined as cranial extension of the main pulmonary artery. Angiograms were reviewed for the presence of pulmonary artery diverticulum, its location, and other associated abnormalities.
Results
Twenty patients in the selected groups had cardiac catheterization during this period. Twelve patients were diagnosed with WS.
Williams Syndrome Group
Mean age at time of catheterization in the WS group was 5 years (range: 9 months to 35 years) (Table 1). Eleven patients with WS had SVAS (91%) and nine patients (81%) had PPS. Two patients had valvar pulmonary stenosis as well. None of the patients had right-sided aortic arch and no other intracardiac abnormalities were documented in these patients. Eight patients had clinical features suggestive of WS.
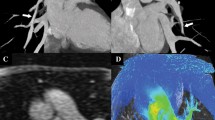
All the patients in this group with PPS had pulmonary diverticulum, which was located at the bifurcation of the pulmonary arteries (Figs. 1, 2) All the patients with supravalve aortic stenosis needed surgery for relief of stenosis. Two patients needed reconstruction of branch pulmonary arteries. One had reconstruction of the right pulmonary artery, while two children needed balloon dilatation of branch pulmonary arteries.
Non-Williams-Syndrome Group
The majority of patients in this group did not have an identifiable dysmorphic syndrome or genetic abnormality (Table 2). Alagille syndrome was present in two patients, and Noonan syndrome in one patient. Mean age at catheterization was 8 years (range: 1–26 years). All of them had PPS and one had valvar pulmonary stenosis as well. No other intracardiac abnormalities were detected. None of the patients in this group had pulmonary artery diverticulum.
Discussion
WS, first described by Williams and associates [12] and Beuren and coworkers [1] in the early 1960s, is a disorder that includes a spectrum of congenital cardiovascular anomalies [13]. Advances in genetic mapping have linked WS and variations of familial SVAS to the deletion or defect of the elastin gene at 7q11 [6]. Cardiovascular disease, particularly an arteriopathy consisting of stenosis of medium-sized and large arteries, is the hallmark of WS. Cardiovascular abnormalities occur in almost 80% of cases of WS [4]. SVAS is the most common cardiac anomaly. Other abnormalities include pulmonary artery stenosis, aortic hypoplasia, coarctation of the aorta, mitral valve prolapse, and septal defects. PPS is associated with various inherited and acquired conditions including maternal rubella, Alagille syndrome, cutis laxa, Noonan syndrome, Ehlers-Danlos syndrome, and WS. PPS can cause obstruction at the level of the main pulmonary artery, at its bifurcation, or at the more distal branches. PPS may occur at a single level, but multiple sites of obstruction are more common.
Pulmonary artery diverticulum (finding of cranial extension of the main pulmonary artery after the bifurcation) has not been described in association with PPS previously, but it was consistently seen in all our patients with WS and PPS, irrespective of age. We use the term diverticulum cautiously, without the histological evidence of pulmonary artery anatomy, in the absence of a better term. Even though one of the differential diagnoses could be persistence of the pulmonary end of an arterial duct (PDA), we have not found any evidence to suggest that there is a higher incidence of PDA in WS [2, 4].
None of the patients with other causes of PPS showed the presence of diverticulum. A ‘pulmonary diverticulum’ has previously been described in association with tetralogy of Fallot but it has not been described as a consistent feature. Matsuyama et al. reviewed 110 angiographies of the tetralogy of Fallot patients specifically for pulmonary diverticulum [9] and found it to be associated with 48% in the right-sided aortic arch and 25.9% in the left-sided aortic arch. They suggested regression of the fourth and sixth brachial arteries as a possible etiology for PA diverticulum, especially because of frequent association with right-sided aortic arch.
Loss of an ELN allele is the single most important genetic change responsible for the cardiovascular problems of WS. Reduced and abnormal elastin content in the media of developing vessels leading to recurrent injury and fibrosis is a postulated mechanism for the development of PPS [10]. Intravascular ultrasound study has demonstrated severe wall thickening involving the endothelial, medial, and adventitial layers with secondary luminal narrowing. Vascular inelasticity may increase hemodynamic stress to the endothelium, leading to intimal proliferation of smooth muscle and fibroblasts causing fibrosis and luminal narrowing of the origin of the branch PAs. This may cause direct transmission of RV pressure on the main pulmonary artery, leading to continued cranial expansion resulting in the ‘diverticulum.’ An embryological origin or persistence of the pulmonary end of a PDA may be another explanation for the diverticulum.
Two patients in our population had a normal IQ and normal phenotype, suggesting the genotypic variability which has been identified with smaller chromosomal deletions as suggested previously [11]. The observation of a normal phenotype in some of our patients with PPS and pulmonary artery diverticulum highlights the importance of screening all patients with diverticulum for specific genetic testing. The presence of branch PA stenosis in combination with SVAS makes this condition more sinister, and pulmonary angiogram must be done in patients with SVAS to rule out PPS.
Asymptomatic pulmonary arterial stenosis associated with subsystemic right ventricular pressure has a favorable long-term prognosis, with eventual spontaneous growth of the pulmonary arterial bed; many patients require no further intervention. Symptomatic patients with suprasystemic right ventricular pressures, on the other hand, require intervention to prolong life and improve quality of life. Surgical treatment is feasible if the proximal branches are involved, but more peripheral stenosis requires percutaneous balloon angioplasty. We were unable to determine the natural course of these diverticulae. Only two patients underwent repeat catheterization for ballooning of branch pulmonary arteries, and there was no change in their dimensions. Moreover, it is unclear whether these diverticula regress if PPS regresses with passage of time [8].
Conclusion
Pulmonary artery diverticulum is a consistent feature in patients with WS. This is the first published report of the association, to the best of our knowledge. Pulmonary artery diverticulum is pathognomonic of WS even in the absence of typical phenotypic features, and we recommend specific genetic testing in any patient with angiographic evidence of a pulmonary ‘diverticulum.’
References
Beuren AJ, Harmjanz D (1962) Supravalvular aortic stenosis in association with mental retardation and certain facial appearance. Circulation 26:1235–1240
Bruno E, Rossi N, Thüer O, Córdoba R, Alday LE (2003) Cardiovascular findings, and clinical course, in patients with Williams syndrome. Cardiol Young 13:532–536
Conway EE Jr, Noonan J, Marion RW, Steeg CN (1990) Myocardial infarction leading to sudden death in the Williams syndrome: report of three cases. J Pediatr 117:593–595
Del Pasqua A, Rinelli G, Toscano A, Iacobelli R, Digilio C, Marino B, Saffirio C, Mondillo S, Pasquini L, Sanders SP, de Zorzi A (2009) New findings concerning cardiovascular manifestations emerging from long-term follow-up of 150 patients with the Williams-Beuren-Beuren syndrome. Cardiol Young 19:563–567
Geggel RL, Gauvreau K, Lock JE (2001) Balloon dilation angioplasty of peripheral pulmonary stenosis associated with Williams syndrome. Circulation 103:2165–2170
Joyce CA, Zorich B, Pike SJ, Barber JC, Dennis NR (1996) Williams-Beuren syndrome: phenotypic variability and deletions of chromosomes 7, 11, and 22 in a series of 52 patients. J Med Genet 33:986–992
Kececioglu D, Kotthoff S, Vogt J (1993) Williams-Beurens syndrome: a 30-year followup of natural and postoperative course. Eur Heart J 14:1458–1464
Pham PP, Moller JH, Hills C, Larson V, Pyles L (2009) Cardiac catheterization and operative outcomes from a multicenter consortium for children with williams syndrome. Pediatr Cardiol 30:9–14
Matsuyama S, Watanabe T, Watabe T, Kuribayashi Y, Tina T, Shohtsu A, Osano M, Inoue T (1977) Pulmonary artery diverticulum associated with tetralogy of Fallot. Tokai J Exp Clin Med 2:113–121
Pober BR, Johnson M, Urban Z (2008) Mechanisms and treatment of cardiovascular disease in Williams-Beuren syndrome. J Clin Invest 118:1606–1615
Tassabehji M (2003) Williams–Beuren syndrome: a challenge for genotype–phenotype correlations. Hum Mol Genet 12:R229–R237
Williams JCP, Love JB (1961) Supravalvular aortic stenosis. Circulation 21:1311–1318
Wessel A, Pankau R, Kececioglu D, Ruschewski W, Bursch JH (1994) Three decades of follow-up of aortic and pulmonary vascular lesions in the Williams-Beuren syndrome. Am J Med Genet 52:297–301
Zalzstein E, Moes CAF, Musewe NN, Freedom RM (1991) Spectrum of cardiovascular anomalies in Williams-Beuren syndrome. Pediatr Cardiol 12:219–223
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahmad, Z., Vettukattil, J. Pulmonary Artery Diverticulum: An Angiographic Marker for Williams Syndrome. Pediatr Cardiol 31, 611–614 (2010). https://doi.org/10.1007/s00246-010-9644-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-010-9644-6