Abstract
Objective
To compare performances in diagnosing intertrochanteric hip fractures from proximal femoral radiographs between a convolutional neural network and orthopedic surgeons.
Materials and methods
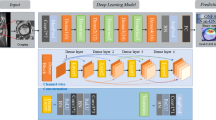
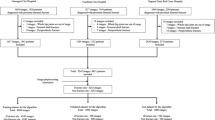
In total, 1773 patients were enrolled in this study. Hip plain radiographs from these patients were cropped to display only proximal fractured and non-fractured femurs. Images showing pseudarthrosis after femoral neck fracture and those showing artificial objects were excluded. This yielded a total of 3346 hip images (1773 fractured and 1573 non-fractured hip images) that were used to compare performances between the convolutional neural network and five orthopedic surgeons.
Results
The convolutional neural network and orthopedic surgeons had accuracies of 95.5% (95% CI = 93.1–97.6) and 92.2% (95% CI = 89.2–94.9), sensitivities of 93.9% (95% CI = 90.1–97.1) and 88.3% (95% CI = 83.3–92.8), and specificities of 97.4% (95% CI = 94.5–99.4) and 96.8% (95% CI = 95.1–98.4), respectively.
Conclusions
The performance of the convolutional neural network exceeded that of orthopedic surgeons in detecting intertrochanteric hip fractures from proximal femoral radiographs under limited conditions. The convolutional neural network has a significant potential to be a useful tool for screening for fractures on plain radiographs, especially in the emergency room, where orthopedic surgeons are not readily available.





Similar content being viewed by others
References
Jamaludin A, Kadir T, Zisserman A. SpineNet: automated classification and evidence visualization in spinal MRIs. Med Image Anal. 2017;41:63–73.
Jamaludin A, Lootus M, Kadir T, et al. Genodisc consortium. ISSLS prize in bioengineering science 2017: automation of reading of radiological features from magnetic resonance images (MRIs) of the lumbar spine without human intervention is comparable with an expert radiologist. Eur Spine J. 2017;26(5):1374–83.
Olczak J, Fahlberg N, Maki A, et al. Artificial intelligence for analyzing orthopedic trauma radiography. Acta Orthop. 2017;88(6):581–6.
Lee H, Tajmir S, Lee J, et al. Fully automated deep learning system for bone age assessment. J Digit Imaging. 2017;30(4):427–41.
Simonyan K, Zisserman A. Very deep convolutional networks for large-scale image recognition. arXiv [Internet]. 2014 [cited 2017 Dec 10] Available from: https://arxiv.org/abs/1409.1556
Kim DH, MacKinnon T. Artificial intelligence in fracture detection: transfer learning from deep convolutional neural networks. Clin Radiol. 2018;73(5):439–45.
Chung SW, Han SS, Lee JW, et al. Automated detection and classification of the proximal humerus fracture by using deep learning algorithm. Acta Orthop. 2018; https://doi.org/10.1080/17453674.2018.1453714.
Hagino H, Endo N, Harada A, et al. Survey of hip fractures in Japan: recent trends in prevalence and treatment. J Orthop Sci. 2017;22(5):909–14.
Pejic A, Hansson S, Rogmark C. Magnetic resonance imaging for verifying hip fracture diagnosis why, when and how? Injury. 2017;48(3):687–91.
Abadi M, Agarwal A, Barham P, et al. TensorFlow: large-scale machine learning on heterogeneous distributed systems. arXiv [Internet]. 2016 [cited 2017 Dec 10] Available from: https://arxiv.org/abs/1603.04467
No authors listed. TesnorFlow-Slim image classification model library. [Internet]. [cited 2017 Dec 10] Available from: https://github.com/tensorflow/models/tree/master/research/slim
Shin HC, Roth HR, Gao M, et al. Deep convolutional neural networks for computer-aided detection: CNN architectures, dataset characteristics and transfer learning. IEEE Trans Med Imaging. 2016;35(5):1285–98.
Geron A. Training deep neural network. In: Geron A, editor. Hands-on machine learning with scikit-learn & TensorFlow. Sebastopol: O’Reilly Media; 2017. p. 275–312.
No authors listed. tf.keras.preprocessing.image.ImageDataGenerator. [Internet]. [cited 2017 Dec 10] Available from: https://www.tensorflow.org/api_docs/python/tf/keras/preprocessing/image/ImageDataGenerator
Kingma DP, Ba J. Adam: a method for stochastic optimization. arXiv [Internet]. 2014 [cited 2017 Dec 10] Available from: https://arxiv.org/abs/1412.6980
Dinah AF. Sequential hip fractures in elderly patients. Injury. 2002;33(5):393–4.
Baumgaertner MR, Higgins TF. Femoral neck fractures. In: Bucholz BW, Heckman JD, editors. Rockwood and Green’s fractures in adults. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2001. p. 1579–634.
Acknowledgements
The authors express their appreciation to Kamimura K, Fujita Y, and Wakui J for reviewing images and would like to thank Editage (www.editage.jp) for English language editing.
Funding
The study was conducted without any external funding or grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Urakawa, T., Tanaka, Y., Goto, S. et al. Detecting intertrochanteric hip fractures with orthopedist-level accuracy using a deep convolutional neural network. Skeletal Radiol 48, 239–244 (2019). https://doi.org/10.1007/s00256-018-3016-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-018-3016-3