Abstract
Purpose
The purpose of the study is to assess the contribution of 18F-fluoro-ethyl-tyrosine (18F-FET) positron emission tomography (PET) in the delineation of gross tumor volume (GTV) in patients with high-grade gliomas compared with magnetic resonance imaging (MRI) alone.
Materials and methods
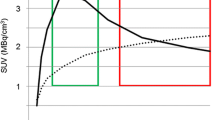
The study population consisted of 18 patients with high-grade gliomas. Seven image segmentation techniques were used to delineate 18F-FET PET GTVs, and the results were compared to the manual MRI-derived GTV (GTVMRI). PET image segmentation techniques included manual delineation of contours (GTVman), a 2.5 standardized uptake value (SUV) cutoff (GTV2.5), a fixed threshold of 40% and 50% of the maximum signal intensity (GTV40% and GTV50%), signal-to-background ratio (SBR)-based adaptive thresholding (GTVSBR), gradient find (GTVGF), and region growing (GTVRG). Overlap analysis was also conducted to assess geographic mismatch between the GTVs delineated using the different techniques.
Results
Contours defined using GTV2.5 failed to provide successful delineation technically in three patients (18% of cases) as SUVmax < 2.5 and clinically in 14 patients (78% of cases). Overall, the majority of GTVs defined on PET-based techniques were usually smaller than GTVMRI (67% of cases). Yet, PET detected frequently tumors that are not visible on MRI and added substantially tumor extension outside the GTVMRI in six patients (33% of cases).
Conclusions
The selection of the most appropriate 18F-FET PET-based segmentation algorithm is crucial, since it impacts both the volume and shape of the resulting GTV. The 2.5 SUV isocontour and GF segmentation techniques performed poorly and should not be used for GTV delineation. With adequate setting, the SBR-based PET technique may add considerably to conventional MRI-guided GTV delineation.






Similar content being viewed by others
References
Omuro AM, Delattre JY. Editorial: what is new in the treatment of gliomas? Curr Opin Neurol 2007;20:704–7.
DeAngelis LM. Brain tumors. N Engl J Med 2001;344:114–23.
Popperl G, Tatsch K, Kreth FW, Tonn JC. Brain tumors. Recent Results Cancer Res 2008;170:33–47.
Chamberlain MC, Murovic JA, Levin VA. Absence of contrast enhancement on CT brain scans of patients with supratentorial malignant gliomas. Neurology 1988;38:1371–4.
Rachinger W, Goetz C, Popperl G, et al. Positron emission tomography with O-(2-[18F]fluoroethyl)-l-tyrosine versus magnetic resonance imaging in the diagnosis of recurrent gliomas. Neurosurgery 2005;57:505–11.
Pauleit D, Floeth F, Hamacher K, et al. O-(2-[18F]fluoroethyl)-l-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain 2005;128:678–87.
Chen W. Clinical applications of PET in brain tumors. J Nucl Med 2007;48:1468–81.
Jager PL, Vaalburg W, Pruim J, de Vries EG, Langen KJ, Piers DA. Radiolabeled amino acids: basic aspects and clinical applications in oncology. J Nucl Med 2001;42:432–45.
Heiss P, Mayer S, Herz M, Wester HJ, Schwaiger M, Senekowitsch-Schmidtke R. Investigation of transport mechanism and uptake kinetics of O-(2-[18F]fluoroethyl)-l-tyrosine in vitro and in vivo. J Nucl Med 1999;40:1367–73.
Spaeth N, Wyss MT, Weber B, et al. Uptake of 18F-fluorocholine, 18F-fluoroethyl-l-tyrosine, and 18F-FDG in acute cerebral radiation injury in the rat: implications for separation of radiation necrosis from tumor recurrence. J Nucl Med 2004;45:1931–8.
Popperl G, Goldbrunner R, Gildehaus FJ, et al. O-(2-[18F]fluoroethyl)-l-tyrosine PET for monitoring the effects of convection-enhanced delivery of paclitaxel in patients with recurrent glioblastoma. Eur J Nucl Med Mol Imaging 2005;32:1018–25.
Mehrkens JH, Popperl G, Rachinger W, et al. The positive predictive value of O-(2-[(18)F]fluoroethyl)-l-tyrosine (FET) PET in the diagnosis of a glioma recurrence after multimodal treatment. J Neurooncol 2008;88:27–35.
Pauleit D, Floeth F, Tellmann L, et al. Comparison of O-(2–18F-fluoroethyl)-l-tyrosine PET and 3-123I-iodo-alpha-methyl-l-tyrosine SPECT in brain tumors. J Nucl Med 2004;45:374–81.
Gregoire V, Haustermans K, Geets X, Roels S, Lonneux M. PET-based treatment planning in radiotherapy: A new standard? J Nucl Med 2007;48:68S–77S.
Boudraa A, Zaidi H. Image segmentation techniques in nuclear medicine imaging. In: Zaidi H, editor. Quantitative analysis of nuclear medicine images. New York: Springer; 2006. p. 308–57.
van Baardwijk A, Baumert BG, Bosmans G, et al. The current status of FDG-PET in tumour volume definition in radiotherapy treatment planning. Cancer Treat Rev 2006;32:245–60.
Erdi Y, Mawlawi O, Larson S, et al. Segmentation of lung lesion volume by adaptive positron emission tomography image thresholding. radioimmunodetection and radioimmunotherapy of cancer, Princeton, New Yersey, 1997:2505–2509.
Daisne JF, Sibomana M, Bol A, Doumont T, Lonneux M, Gregoire V. Tri-dimensional automatic segmentation of PET volumes based on measured source-to-background ratios: influence of reconstruction algorithms. Radiother Oncol 2003;69:247–50.
Ciernik IF, Dizendorf E, Baumert BG, et al. Radiation treatment planning with an integrated positron emission and computer tomography (PET/CT): a feasibility study. Int J Radiat Oncol Biol Phys 2003;57:853–63.
Yaremko B, Riauka T, Robinson D, et al. Thresholding in PET images of static and moving targets. Phys Med Biol 2005;50:5969–82.
Nestle U, Kremp S, Schaefer-Schuler A, et al. Comparison of different methods for delineation of 18F-FDG PET-positive tissue for target volume definition in radiotherapy of patients with non-Small cell lung cancer. J Nucl Med 2005;46:1342–8.
Drever L, Roa W, McEwan A, Robinson D. Iterative threshold segmentation for PET target volume delineation. Med Phys 2007;34:1253–65.
Schinagl DA, Vogel WV, Hoffmann AL, van Dalen JA, Oyen WJ, Kaanders JH. Comparison of five segmentation tools for 18F-fluoro-deoxy-glucose-positron emission tomography-based target volume definition in head and neck cancer. Int J Radiat Oncol Biol Phys 2007;69:1282–9.
Hatt M, Lamare F, Boussion N, et al. Fuzzy hidden Markov chains segmentation for volume determination and quantitation in PET. Phys Med Biol 2007;52:3467–91.
Montgomery D, Amira A, Zaidi H. Fully automated segmentation of oncological PET volumes using a combined multiscale and statistical model. Med Phys 2007;34:722–36.
Hong R, Halama J, Bova D, Sethi A, Emami B. Correlation of PET standard uptake value and CT window-level thresholds for target delineation in CT-based radiation treatment planning. Int J Radiat Oncol Biol Phys 2007;67:720–6.
Jannin P, Fitzpatrick JM, Hawkes DJ, Pennec X, Shahidi R, Vannier MW. Validation of medical image processing in image-guided therapy. IEEE Trans Med Imaging 2002;21:1445–9.
Pauleit D, Floeth F, Herzog H, et al. Whole-body distribution and dosimetry of O-(2-[18F]fluoroethyl)-l-tyrosine. Eur J Nucl Med Mol Imaging 2003;30:519–24.
Graves EE, Quon A, Loo BW Jr. RT_Image: an open-source tool for investigating PET in radiation oncology. Technol Cancer Res Treat 2007;6:111–21.
Jentzen W, Freudenberg L, Eising EG, Heinze M, Brandau W, Bockisch A. Segmentation of PET volumes by iterative image thresholding. J Nucl Med 2007;48:108–14.
Basu S, Zaidi H, Houseni M, et al. Novel quantitative techniques for assessing regional and global function and structure based on modern imaging modalities: Implications for normal variation, aging and diseased states. Sem Nucl Med 2007;37:223–39.
Plotkin M, Gneveckow U, Meier-Hauff K, et al. 18F-FET PET for planning of thermotherapy using magnetic nanoparticles in recurrent glioblastoma. Int J Hyperthermia 2006;22:319–25.
Floeth FW, Pauleit D, Sabel M, et al. 18F-FET PET differentiation of ring-enhancing brain lesions. J Nucl Med 2006;47:776–82.
Langen KJ, Floeth FW, Stoffels G, Hamacher K, Coenen HH, Pauleit D. Improved diagnostics of cerebral gliomas using FET PET. Z Med Phys 2007;17:237–41.
Mosskin M, von Holst H, Bergstrom M, et al. Positron emission tomography with 11C-methionine and computed tomography of intracranial tumours compared with histopathologic examination of multiple biopsies. Acta Radiol 1987;28:673–81.
Grosu AL, Weber WA, Franz M, et al. Reirradiation of recurrent high-grade gliomas using amino acid PET (SPECT)/CT/MRI image fusion to determine gross tumor volume for stereotactic fractionated radiotherapy. Int J Radiat Oncol Biol Phys 2005;63:511–9.
Grosu AL, Weber WA, Riedel E, et al. l-(methyl-11C) methionine positron emission tomography for target delineation in resected high-grade gliomas before radiotherapy. Int J Radiat Oncol Biol Phys 2005;63:64–74.
Mahasittiwat P, Mizoe JE, Hasegawa A, et al. l-[METHYL-(11)C] methionine positron emission tomography for target delineation in malignant gliomas: impact on results of carbon ion radiotherapy. Int J Radiat Oncol Biol Phys 2008;70:515–22.
Daisne JF, Dupers T, Weygand B, et al. Tumor volume in pharyngolaryngeal squamous cell carcinoma: comparison at CT, MR imaging, and FDG PET and validation with surgical specimen. Radiology 2004;233:93–100.
Weber WA, Wester HJ, Grosu AL, et al. O-(2-[18F]fluoroethyl)-l-tyrosine and l-[methyl-11C]methionine uptake in brain tumours: initial results of a comparative study. Eur J Nucl Med 2000;27:542–9.
Pirzkall A, Li X, Oh J, et al. 3D MRSI for resected high-grade gliomas before RT: tumor extent according to metabolic activity in relation to MRI. Int J Radiat Oncol Biol Phys 2004;59:126–37.
Zhang YJ. A survey on evaluation methods for image segmentation. Patt Recogn Letters 1996;29:1335–46.
Acknowledgments
This work was supported by the Swiss National Science Foundation under grant No. 3152A0-102143, the Indo Swiss Bilateral Research Initiative (ISBRI) supported by the Swiss State Secretariat for Education and Research under grant No. AP24, and the foundation Cellex International. This paper is dedicated to the memory of Prof. Bruce Hasegawa (Department of Radiology, UCSF), a brilliant scientist and true friend who sadly passed away last summer.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vees, H., Senthamizhchelvan, S., Miralbell, R. et al. Assessment of various strategies for 18F-FET PET-guided delineation of target volumes in high-grade glioma patients. Eur J Nucl Med Mol Imaging 36, 182–193 (2009). https://doi.org/10.1007/s00259-008-0943-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-008-0943-6