Abstract
Purpose
Targeting fibroblast activation protein (FAP) is a new diagnostic approach allowing the visualization of tumor stroma. Here, we applied FAP-specific PET imaging to gliomas. We analyzed the target affinity and specificity of two FAP ligands (FAPI-02 and FAPI-04) in vitro, and the pharmacokinetics and biodistribution in mice in vivo. Clinically, we used 68Ga-labeled FAPI-02/04 for PET imaging in 18 glioma patients (five IDH-mutant gliomas, 13 IDH-wildtype glioblastomas).
Methods
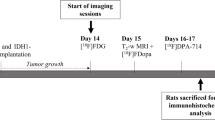
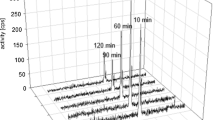
For binding studies with 177Lu-radiolabeled FAPI-02/04, we used the glioblastoma cell line U87MG, FAP-transfected fibrosarcoma cells, and CD26-transfected human embryonic kidney cells. For pharmacokinetic and biodistribution studies, U87MG-xenografted mice were injected with 68Ga-labeled compounds followed by small-animal PET imaging and 177Lu-labeled FAPI-02/04, respectively. Clinical PET/CT scans were performed 30 min post intravenous administration of 68Ga-FAPI-02/04. PET and MRI scans were co-registrated. Immunohistochemistry was done on 14 gliomas using a FAP-specific antibody.
Results
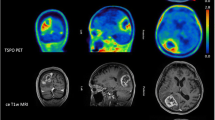
FAPI-02 and FAPI-04 showed high binding specificity to FAP. FAPI-04 demonstrated higher tumor accumulation and delayed elimination compared with FAPI-02 in preclinical studies. IDH-wildtype glioblastomas and grade III/IV, but not grade II, IDH-mutant gliomas showed elevated tracer uptake. In glioblastomas, we observed spots with increased uptake in projection on contrast-enhancing areas. Immunohistochemistry showed FAP-positive cells with mainly elongated cell bodies and perivascular FAP-positive cells in glioblastomas and an anaplastic IDH-mutant astrocytoma.
Conclusions
Using FAP-specific PET imaging, increased tracer uptake in IDH-wildtype glioblastomas and high-grade IDH-mutant astrocytomas, but not in diffuse astrocytomas, may allow non-invasive distinction between low-grade IDH-mutant and high-grade gliomas. Therefore, FAP-specific imaging in gliomas may be useful for follow-up studies although further clinical evaluation is required.




Similar content being viewed by others
References
Liao Z, Tan ZW, Zhu P, Tan NS. Cancer-associated fibroblasts in tumor microenvironment - accomplices in tumor malignancy. Cell Immunol. 2018. https://doi.org/10.1016/j.cellimm.2017.12.003.
Santi A, Kugeratski FG, Zanivan S. Cancer associated fibroblasts: the architects of stroma remodeling. Proteomics. 2018;18:e1700167. https://doi.org/10.1002/pmic.201700167.
Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16:582–98. https://doi.org/10.1038/nrc.2016.73.
McCarthy JB, El-Ashry D, Turley EA. Hyaluronan, cancer-associated fibroblasts and the tumor microenvironment in malignant progression. Front Cell Dev Biol. 2018;6:48. https://doi.org/10.3389/fcell.2018.00048.
Lai D, Ma L, Wang F. Fibroblast activation protein regulates tumor-associated fibroblasts and epithelial ovarian cancer cells. Int J Oncol. 2012;41:541–50. https://doi.org/10.3892/ijo.2012.1475.
Koczorowska MM, Tholen S, Bucher F, Lutz L, Kizhakkedathu JN, De Wever O, et al. Fibroblast activation protein-alpha, a stromal cell surface protease, shapes key features of cancer associated fibroblasts through proteome and degradome alterations. Mol Oncol. 2016;10:40–58. https://doi.org/10.1016/j.molonc.2015.08.001.
Loktev A, Lindner T, Mier W, Debus J, Altmann A, Jager D, et al. A new method for tumor imaging by targeting cancer associated fibroblasts. J Nucl Med. 2018. https://doi.org/10.2967/jnumed.118.210435.
Lindner T, Loktev A, Altmann A, Giesel F, Kratochwil C, Debus J, et al. Development of quinoline based theranostic ligands for the targeting of fibroblast activation protein. J Nucl Med. 2018. https://doi.org/10.2967/jnumed.118.210443.
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–20. https://doi.org/10.1007/s00401-016-1545-1.
Langen KJ, Stoffels G, Filss C, Heinzel A, Stegmayr C, Lohmann P, et al. Imaging of amino acid transport in brain tumours: positron emission tomography with O-(2-[(18)F]fluoroethyl)-L-tyrosine (FET). Methods. 2017;130:124–34. https://doi.org/10.1016/j.ymeth.2017.05.019.
Palanichamy K, Chakravarti A. Diagnostic and prognostic significance of methionine uptake and methionine positron emission tomography imaging in gliomas. Front Oncol. 2017;7:257. https://doi.org/10.3389/fonc.2017.00257.
Hoffman RM. L-[methyl-(11)C] Methionine-positron-emission tomography (MET-PET). Methods Mol Biol. 1866;2019:267–71. https://doi.org/10.1007/978-1-4939-8796-2_20.
Bell C, Dowson N, Puttick S, Gal Y, Thomas P, Fay M, et al. Increasing feasibility and utility of (18)F-FDOPA PET for the management of glioma. Nucl Med Biol. 2015;42:788–95. https://doi.org/10.1016/j.nucmedbio.2015.06.001.
Fueger BJ, Czernin J, Cloughesy T, Silverman DH, Geist CL, Walter MA, et al. Correlation of 6-18F-fluoro-L-dopa PET uptake with proliferation and tumor grade in newly diagnosed and recurrent gliomas. J Nucl Med. 2010;51:1532–8. https://doi.org/10.2967/jnumed.110.078592.
Busek P, Balaziova E, Matrasova I, Hilser M, Tomas R, Syrucek M, et al. Fibroblast activation protein alpha is expressed by transformed and stromal cells and is associated with mesenchymal features in glioblastoma. Tumour Biol. 2016;37:13961–71. https://doi.org/10.1007/s13277-016-5274-9.
Mentlein R, Hattermann K, Hemion C, Jungbluth AA, Held-Feindt J. Expression and role of the cell surface protease seprase/fibroblast activation protein-alpha (FAP-alpha) in astroglial tumors. Biol Chem. 2011;392:199–207. https://doi.org/10.1515/BC.2010.119.
Floca R. MatchPoint: on bridging the innovation gap between algorithmic research and clinical use in image registration. World Congress on Medical Physics and Biomedical Engineering, September 7–12, 2009, Munich, Germany. 2009:1105–8.
Nolden M, Zelzer S, Seitel A, Wald D, Muller M, Franz AM, et al. The medical imaging interaction toolkit: challenges and advances : 10 years of open-source development. Int J Comput Assist Radiol Surg. 2013;8:607–20. https://doi.org/10.1007/s11548-013-0840-8.
RCoreTeam. R: A language and environment for statistical computing. R Foundation for Statistical Computing. 2017.
Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez J-C, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:1.
Kelly T. Fibroblast activation protein-alpha and dipeptidyl peptidase IV (CD26): cell-surface proteases that activate cell signaling and are potential targets for cancer therapy. Drug Resist Updat. 2005;8:51–8. https://doi.org/10.1016/j.drup.2005.03.002.
Albert NL, Weller M, Suchorska B, Galldiks N, Soffietti R, Kim MM, et al. Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro-Oncology. 2016;18:1199–208. https://doi.org/10.1093/neuonc/now058.
Albert NL, Winkelmann I, Suchorska B, Wenter V, Schmid-Tannwald C, Mille E, et al. Early static (18)F-FET-PET scans have a higher accuracy for glioma grading than the standard 20-40 min scans. Eur J Nucl Med Mol Imaging. 2016;43:1105–14. https://doi.org/10.1007/s00259-015-3276-2.
Jansen NL, Graute V, Armbruster L, Suchorska B, Lutz J, Eigenbrod S, et al. MRI-suspected low-grade glioma: is there a need to perform dynamic FET PET? Eur J Nucl Med Mol Imaging. 2012;39:1021–9. https://doi.org/10.1007/s00259-012-2109-9.
Pauleit D, Floeth F, Hamacher K, Riemenschneider MJ, Reifenberger G, Muller HW, et al. O-(2-[18F]fluoroethyl)-L-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain. 2005;128:678–87. https://doi.org/10.1093/brain/awh399.
Popperl G, Kreth FW, Mehrkens JH, Herms J, Seelos K, Koch W, et al. FET PET for the evaluation of untreated gliomas: correlation of FET uptake and uptake kinetics with tumour grading. Eur J Nucl Med Mol Imaging. 2007;34:1933–42. https://doi.org/10.1007/s00259-007-0534-y.
Rohrich M, Huang K, Schrimpf D, Albert NL, Hielscher T, von Deimling A, et al. Integrated analysis of dynamic FET PET/CT parameters, histology, and methylation profiling of 44 gliomas. Eur J Nucl Med Mol Imaging. 2018;45:1573–84. https://doi.org/10.1007/s00259-018-4009-0.
Verger A, Stoffels G, Bauer EK, Lohmann P, Blau T, Fink GR, et al. Static and dynamic (18)F-FET PET for the characterization of gliomas defined by IDH and 1p/19q status. Eur J Nucl Med Mol Imaging. 2018;45:443–51. https://doi.org/10.1007/s00259-017-3846-6.
Di Carlo DT, Duffau H, Cagnazzo F, Benedetto N, Morganti R, Perrini P. IDH wild-type WHO grade II diffuse low-grade gliomas. A heterogeneous family with different outcomes. Systematic review and meta-analysis. Neurosurg Rev. 2018. https://doi.org/10.1007/s10143-018-0996-3.
Reuss DE, Mamatjan Y, Schrimpf D, Capper D, Hovestadt V, Kratz A, et al. IDH mutant diffuse and anaplastic astrocytomas have similar age at presentation and little difference in survival: a grading problem for WHO. Acta Neuropathol. 2015;129:867–73. https://doi.org/10.1007/s00401-015-1438-8.
Pallud J, Capelle L, Taillandier L, Fontaine D, Mandonnet E, Guillevin R, et al. Prognostic significance of imaging contrast enhancement for WHO grade II gliomas. Neuro-Oncology. 2009;11:176–82. https://doi.org/10.1215/15228517-2008-066.
Freyschlag CF, Krieg SM, Kerschbaumer J, Pinggera D, Forster MT, Cordier D, et al. Imaging practice in low-grade gliomas among European specialized centers and proposal for a minimum core of imaging. J Neuro-Oncol. 2018;139:699–711. https://doi.org/10.1007/s11060-018-2916-3.
Gempt J, Bette S, Ryang YM, Buchmann N, Peschke P, Pyka T, et al. 18F-Fluoro-ethyl-tyrosine positron emission tomography for grading and estimation of prognosis in patients with intracranial gliomas. Eur J Radiol. 2015;84:955–62. https://doi.org/10.1016/j.ejrad.2015.01.022.
Jansen NL, Suchorska B, Wenter V, Eigenbrod S, Schmid-Tannwald C, Zwergal A, et al. Dynamic 18F-FET PET in newly diagnosed astrocytic low-grade glioma identifies high-risk patients. J Nucl Med. 2014;55:198–203. https://doi.org/10.2967/jnumed.113.122333.
Unterrainer M, Schweisthal F, Suchorska B, Wenter V, Schmid-Tannwald C, Fendler WP, et al. Serial 18F-FET PET imaging of primarily 18F-FET-negative glioma: does it make sense? J Nucl Med. 2016;57:1177–82. https://doi.org/10.2967/jnumed.115.171033.
Imani F, Boada FE, Lieberman FS, Davis DK, Deeb EL, Mountz JM. Comparison of proton magnetic resonance spectroscopy with fluorine-18 2-fluoro-deoxyglucose positron emission tomography for assessment of brain tumor progression. J Neuroimaging. 2012;22:184–90. https://doi.org/10.1111/j.1552-6569.2010.00561.x.
Dankbaar JW, Snijders TJ, Robe PA, Seute T, Eppinga W, Hendrikse J, et al. The use of (18)F-FDG PET to differentiate progressive disease from treatment induced necrosis in high grade glioma. J Neuro-Oncol. 2015;125:167–75. https://doi.org/10.1007/s11060-015-1883-1.
Henze M, Mohammed A, Schlemmer HP, Herfarth KK, Hoffner S, Haufe S, et al. PET and SPECT for detection of tumor progression in irradiated low-grade astrocytoma: a receiver-operating-characteristic analysis. J Nucl Med. 2004;45:579–86.
Capper D, Zentgraf H, Balss J, Hartmann C, von Deimling A. Monoclonal antibody specific for IDH1 R132H mutation. Acta Neuropathol. 2009;118:599–601. https://doi.org/10.1007/s00401-009-0595-z.
Hamson EJ, Keane FM, Tholen S, Schilling O, Gorrell MD. Understanding fibroblast activation protein (FAP): substrates, activities, expression and targeting for cancer therapy. Proteomics Clin Appl. 2014;8:454–63. https://doi.org/10.1002/prca.201300095.
Aimes RT, Zijlstra A, Hooper JD, Ogbourne SM, Sit ML, Fuchs S, et al. Endothelial cell serine proteases expressed during vascular morphogenesis and angiogenesis. Thromb Haemost. 2003;89:561–72.
Clavreul A, Etcheverry A, Chassevent A, Quillien V, Avril T, Jourdan ML, et al. Isolation of a new cell population in the glioblastoma microenvironment. J Neuro-Oncol. 2012;106:493–504. https://doi.org/10.1007/s11060-011-0701-7.
Clavreul A, Guette C, Faguer R, Tetaud C, Boissard A, Lemaire L, et al. Glioblastoma-associated stromal cells (GASCs) from histologically normal surgical margins have a myofibroblast phenotype and angiogenic properties. J Pathol. 2014;233:74–88. https://doi.org/10.1002/path.4332.
Trylcova J, Busek P, Smetana K Jr, Balaziova E, Dvorankova B, Mifkova A, et al. Effect of cancer-associated fibroblasts on the migration of glioma cells in vitro. Tumour Biol. 2015;36:5873–9. https://doi.org/10.1007/s13277-015-3259-8.
Funding
This work was funded by the Federal Ministry of Education and Research, grant number 13N 13341.
Author information
Authors and Affiliations
Contributions
Experimental design: AL, MR, TL, AA, and UH; preclinical experiments: AL, TL, AA, and MR; clinical studies: MR, SA, PW, PF, and JD; PET/MRI co-registration studies: DP, MR, and RF; statistical analysis: TH; immunohistochemistry: PEH, JPS, AW, and AVD. All authors have seen and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval of animal experiments
The animal experiments of this work were approved by the local ethics committee and all applicable international, national, and institutional guidelines for the care and use of animals were followed.
Ethical approval of clinical studies
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All patients signed written informed consent. The evaluation was approved by our institutional ethical review board (no. S-016/2018).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuel Röhrich, Anastasia Loktev, Thomas Lindner, and Uwe Haberkorn are co-authors.
This article is part of the Topical Collection on Oncology – Brain
Rights and permissions
About this article
Cite this article
Röhrich, M., Loktev, A., Wefers, A.K. et al. IDH-wildtype glioblastomas and grade III/IV IDH-mutant gliomas show elevated tracer uptake in fibroblast activation protein–specific PET/CT. Eur J Nucl Med Mol Imaging 46, 2569–2580 (2019). https://doi.org/10.1007/s00259-019-04444-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-04444-y