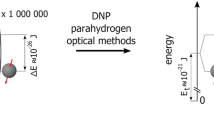
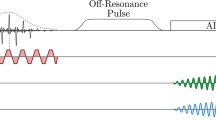
Abstract
The evolution of magnetic resonance imaging (MRI) has been astounding since the early 1980s, and a broad range of applications has emerged. To date, clinical imaging of nuclei other than protons has been precluded for reasons of sensitivity. However, with the recent development of hyperpolarization techniques, the signal from a given number of nuclei can be increased as much as 100,000 times, sufficient to enable imaging of nonproton nuclei. Technically, imaging of hyperpolarized nuclei offers several unique properties, such as complete lack of background signal and possibility for local and permanent destruction of the signal by means of radio frequency (RF) pulses. These properties allow for improved as well as new techniques within several application areas. Diagnostically, the injected compounds can visualize information about flow, perfusion, excretory function, and metabolic status. In this review article, we explain the concept of hyperpolarization and the techniques to hyperpolarize 13C. An overview of results obtained within angiography, perfusion, and catheter tracking is given, together with a discussion of the particular advantages and limitations. Finally, possible future directions of hyperpolarized 13C MRI are pointed out.










Similar content being viewed by others
References
Bloch F, Hansen WW, Packard M (1946) The nuclear induction experiment. Phys Rev 70:474–485
Purcell EM, Torrey HC, Pound RV (1946) Resonance absorption by nuclear magnetic moments in a solid. Phys Rev 69:37–38
Lauterbur PC (1973) Image formation by induced local interactions: examples employing nuclear magnetic resonance. Nature 242:190–191
Shulman RG, Rothman DL (2001) 13C NMR of intermediary metabolism: implications for systemic physiology. Annu Rev Physiol 63:15–48
Albert MS, Balamore D (1998) Development of hyperpolarized noble gas MRI. Nucl Instrum Methods Phys Res A 402:441–453
Albert MS, Cates GD, Driehuys B, Happer W, Saam B, Springer CS Jr, Wishnia A (1994) Biological magnetic resonance imaging using laser-polarized 129Xe. Nature 370:199–201
Middleton H, Black RD, Saam B, Cates GD, Cofer GP, Guenther R, Happer W, Hedlund LW, Johnson GA, Juvan K et al (1995) MR imaging with hyperpolarized 3He gas. Magn Reson Med 33:271–275
Kauczor H, Surkau R, Roberts T (1998) MRI using hyperpolarized noble gases. Eur Radiol 8:820–827
Kauczor HU (2003) Hyperpolarized helium-3 gas magnetic resonance imaging of the lung. Top Magn Reson Imaging 14:223–230
van Beek EJ, Wild JM, Kauczor HU, Schreiber W, Mugler JP III, de Lange EE (2004) Functional MRI of the lung using hyperpolarized 3-helium gas. J Magn Reson Imaging 20:540–554
Jóhannesson H, Axelsson O, Karlsson M (2004) Transfer of para-hydrogen spin order into polarization by diabatic field cycling. C R Physique 5:315–324
Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K (2003) Increase in signal-to-noise ratio of >10,000 times in liquid-state NMR. Proc Natl Acad Sci U S A 100:10158–10163
Campeau NG, Huston J III, Bernstein MA, Lin C, Gibbs GF (2001) Magnetic resonance angiography at 3.0 Tesla: initial clinical experience. Top Magn Reson Imaging 12:183–204
Ardenkjaer-Larsen JH, Axelsson O, Golman K, Wistrand LG, Hansson G, Leunbach I, Petersson JS (1999) Method of magnetic resonance investigation. International patent application no. WO 99/35508
Frossati G (1998) Polarization of 3He, D2 (and possibly 129Xe) using cryogenic techniques. Nucl Instrum Meth A 402:479–483
Abragam A, Goldman M (1978) Principles of dynamic nuclear polarisation. Rep Prog Phys 41:395–467
Bowers CR, Weitekamp DP (1986) Transformation of symmetrization order to nuclear-spin magnetization by chemical reaction and nuclear magnetic resonance. Phys Rev Lett 57:2645–2648
Bowers CR, Weitekamp DP (1987) Parahydrogen and synthesis allow dramatically enhanced nuclear alignment. J Am Chem Soc 109:5541–5542
Golman K, Axelsson O, Jóhannesson H, Månsson S, Olofsson C, Petersson JS (2001) Parahydrogen-induced polarization in imaging: subsecond 13C angiography. Magn Reson Med 46:1–5
Jóhannesson H, Axelsson O, Karlsson M, Goldman M (2004) Methods to convert para-hydrogen spin order into hetero nuclei polarization for in vivo detection. In: Proc 21st Annual Meeting ESMRMB:144
Prince MR, Yucel EK, Kaufman JA, Harrison DC, Geller SC (1993) Dynamic gadolinium-enhanced three-dimensional abdominal MR arteriography. J Magn Reson Imaging 3:877–881
Merbach A, Tóth É (2001) The chemistry of contrast agents in medical magnetic resonance imaging. John Wiley & Sons, Chichester
Nishimura DG, Macovski A, Pauly JM (1986) Magnetic resonance angiography. IEEE Trans Med Imaging 5:140–151
Maki JH, Chenevert TL, Prince MR (1996) Three-dimensional contrast-enhanced MR angiography. Top Magn Reson Imaging 8:322–344
Golman K, Ardenkjaer-Larsen JH, Petersson JS, Månsson S, Leunbach I (2003) Molecular imaging with endogenous substances. Proc Natl Acad Sci U S A 100:10435–10439
Golman K, Ardenkjaer-Larsen JH, Svensson J, Axelsson O, Hansson G, Hansson L, Johannesson H, Leunbach I, Månsson S, Petersson JS, Pettersson G, Servin R, Wistrand LG (2002) 13C-angiography. Acad Radiol 9(Suppl 2):S507–S510
Markstaller K, Eberle B, Schreiber WG, Weiler N, Thelen M, Kauczor HU (2000) Flip angle considerations in (3)helium-MRI. NMR Biomed 13:190–193
Zhao L, Albert MS (1998) Biomedical imaging using hyperpolarized noble gas MRI: pulse sequence considerations. Nucl Instrum Methods Phys Res A 402:454–460
Svensson J, Månsson S, Johansson E, Petersson JS, Olsson LE (2003) Hyperpolarized 13C MR angiography using trueFISP. Magn Reson Med 50:256–262
Kiselev VG (2001) On the theoretical basis of perfusion measurements by dynamic susceptibility contrast MRI. Magn Reson Med 46:1113–1122
Larsson HB, Fritz-Hansen T, Rostrup E, Sondergaard L, Ring P, Henriksen O (1996) Myocardial perfusion modeling using MRI. Magn Reson Med 35:716–726
Johansson E, Månsson S, Wirestam R, Svensson J, Petersson JS, Golman K, Ståhlberg F (2004) Cerebral perfusion assessment by bolus tracking using hyperpolarized 13C. Magn Reson Med 51:464–472
Johansson E (2003) NMR imaging of flow and perfusion using hyperpolarized nuclei. Thesis, Lund University, Sweden
Johansson E, Magnusson P, Chai C-M, Petersson J, Golman K, Wirestam R, Ståhlberg F (2004) Assessing myocardial perfusion using hyperpolarized 13C. In: Proc 21st Annual Meeting ESMRMB:117
Johansson E, Olsson LE, Mansson S, Petersson JS, Golman K, Ståhlberg F, Wirestam R (2004) Perfusion assessment with bolus differentiation: a technique applicable to hyperpolarized tracers. Magn Reson Med 52:1043–1051
Uematsu H, Ohno Y, Hatabu H (2003) Recent advances in magnetic resonance perfusion imaging of the lung. Top Magn Reson Imaging 14:245–251
West JB, Wagner PD (1997) Ventilation-perfusion relationships. In: Crystal RG, West JB, Barnes PJ, Weibel ER (eds) The lung: scientific foundations. Lippincott Williams & Wilkins, Philadelphia, pp 1693–1709
Kearon C (2003) Diagnosis of pulmonary embolism. CMAJ 168:183–194
Wagner PD, Saltzman HA, West JB (1974) Measurement of continuous distributions of ventilation-perfusion ratios: theory. J Appl Physiol 36:588–599
Robertson HT, Glenny RW, Stanford D, McInnes LM, Luchtel DL, Covert D (1997) High-resolution maps of regional ventilation utilizing inhaled fluorescent microspheres. J Appl Physiol 82:943–953
Edelman RR, Hatabu H, Tadamura E, Li W, Prasad PV (1996) Noninvasive assessment of regional ventilation in the human lung using oxygen-enhanced magnetic resonance imaging. Nat Med 2:1236–1239
Ohno Y, Hatabu H, Takenaka D, Adachi S, Van Cauteren M, Sugimura K (2001) Oxygen-enhanced MR ventilation imaging of the lung: preliminary clinical experience in 25 subjects. Am J Roentgenol 177:185–194
Simon BA, Marcucci C, Fung M, Lele SR (1998) Parameter estimation and confidence intervals for Xe-CT ventilation studies: a Monte Carlo approach. J Appl Physiol 84:709–716
Peters DC, Lederman RJ, Dick AJ, Raman VK, Guttman MA, Derbyshire JA, McVeigh ER (2003) Undersampled projection reconstruction for active catheter imaging with adaptable temporal resolution and catheter-only views. Magn Reson Med 49:216–222
Serfaty JM, Yang X, Aksit P, Quick HH, Solaiyappan M, Atalar E (2000) Toward MRI-guided coronary catheterization: visualization of guiding catheters, guidewires, and anatomy in real time. J Magn Reson Imaging 12:590–594
Wildermuth S, Dumoulin CL, Pfammatter T, Maier SE, Hofmann E, Debatin JF (1998) MR-guided percutaneous angioplasty: assessment of tracking safety, catheter handling and functionality. Cardiovasc Intervent Radiol 21:404–410
Zimmermann-Paul GG, Ladd ME, Pfammatter T, Hilfiker PR, Quick HH, Debatin JF (1998) MR versus fluoroscopic guidance of a catheter/guidewire system: in vitro comparison of steerability. J Magn Reson Imaging 8:1177–1181
Bakker CJ, Hoogeveen RM, Hurtak WF, van Vaals JJ, Viergever MA, Mali WP (1997) MR-guided endovascular interventions: susceptibility-based catheter and near-real-time imaging technique. Radiology 202:273–276
Green JD, Omary RA, Finn JP, Tang R, Li Y, Carr J, Li D (2002) Passive catheter tracking using MRI: comparison of conventional and magnetization-prepared FLASH. J Magn Reson Imaging 16:104–109
Magnusson P, Månsson S, Petersson J, Chai C-M, Hansson G, Johansson E (2004) Passive catheter tracking using MRI and hyperpolarized 13C. In: Proc 21st Annual Meeting ESMRMB:143
Ross B, Michaelis T (1994) Clinical applications of magnetic resonance spectroscopy. Magn Reson Q 10:191–247
Cousins JP (1995) Clinical MR spectroscopy: fundamentals, current applications, and future potential. Am J Roentgenol 164:1337–1347
Henriksen O (1994) MR spectroscopy in clinical research. Acta Radiol 35:96–116
Sonnewald U, Gribbestad IS, Westergaard N, Nilsen G, Unsgard G, Schousboe A, Petersen SB (1994) Nuclear magnetic resonance spectroscopy: biochemical evaluation of brain function in vivo and in vitro. Neurotoxicology 15:579–590
Kety SS, Schmidt CF (1948) The nitrous oxide method for the quantitative determination of cerebral blood flow in man: theory, procedure and normal values. J Clin Invest 27:476–483
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Månsson, S., Johansson, E., Magnusson, P. et al. 13C imaging—a new diagnostic platform. Eur Radiol 16, 57–67 (2006). https://doi.org/10.1007/s00330-005-2806-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-005-2806-x