Abstract
Acute liver failure (ALF)-related encephalopathy was previously characterized by MR spectroscopy of single voxels containing both grey and white matter brain tissue. Quantitative multivoxel MRS was used here to compare grey and white matter brain tissue concentrations of glutamate/glutamine (Glx) and lactate in ALF and associate the results with other liver function parameters. Five pediatric patients with ALF-related encephalopathy and five controls, examined after successful liver transplantation, were examined by brain MRI/MRS. ALF patients had higher Glx and lactate concentrations in brain white matter than controls (Glx + 125%: P < 0.01; lactate + 33%, P < 0.05) and higher Glx in grey matter (Glx + 125%: P < 0.01). Within the group of ALF patients positive correlations were found between grey or white matter lactate concentration and serum ammonia (P < 0.05), and negative correlations between grey or white matter Glx and venous pH (P < 0.001). This is the first study presenting evidence of high Glx levels in both white and grey matter brain tissue in ALF-related encephalopathy. The elevations in CNS Glx and lactate concentrations appear to relate to hepatic detoxification (ammonia, venous pH), rather than to liver parenchymal integrity (aspartate aminotransferase, alanine aminotransferase) or biliary cholestasis (bilirubin, γ-glutamyl transpeptidase, alkaline phosphatase).
Similar content being viewed by others
Introduction
Encephalopathy, a common and potentially life-threatening complication of acute liver failure (ALF) [1, 2], is predominantly a clinical diagnosis. Anatomical MRI frequently does not show abnormalities [3]. Especially in children, encephalopathy can be difficult to assess. Therefore, the quest for additional diagnostic tools for assessing the presence and severity of ALF-related encephalopathy continues.
The recent availability of sensitive diagnostic tests, such as magnetic resonance spectroscopy (MRS), allows us to determine possible relationships between serum indicators for liver function (total and indirect bilirubin, aspartate aminotransferase, alanine aminotransferase) and parameters of CNS physiology in humans in vivo. The main cell metabolites in brain that can now be quantified by MRS are choline, creatine, N-acetylaspartate, glutamate/glutamine (Glx) and lactate. The detection limit of the MRS technique is in the order of 0.1 mM. The power of MRS methodology has improved substantially with the advent of multi-voxel methods allowing for simultaneous assessment of grey and white matter metabolism. Furthermore, technical improvements allow for operator-independent automated postprocessing and quantification of lactate signals. Previous studies indicated increased Glx levels in brains of patients with neonatal jaundice, with hepatic encephalopathy and with ALF [4–7]. In previous documentations of brain MRS results in ALF peak intensities were assessed qualitatively by means of peak intensity ratios in one single voxel containing both grey and white matter tissue [6, 7]. Although cutting edge at that time, the limitations of that approach were that the results obtained in different patients could not be compared in terms of specific metabolites and could not differentiate between grey and white matter. Moreover, the interpretations were based on the assumption that results obtained in one small volume were representative of the brain as a whole. In a recent MRS study of cirrhotic patients with hepatic encephalopathy, changes in MR spectra could be shown only for white matter [8]. Much earlier, regional variations in the obtained MRS metabolite ratios had already been pointed out, suggesting that spectral information from more than one voxel would be useful in the evaluation of patients with hepatic encephalopathy [9].
In the present MRS study we therefore set out to quantify both white and grey matter brain metabolism in pediatric ALF patients with various degrees of encephalopathy, in comparison with a control group of pediatric patients without encephalopathy examined after liver transplantation. A technical improvement compared with previous studies was the use of quantitative chemical shift imaging allowing for direct comparison of curve-fitted metabolite peak areas in arrays of multiple grey and white matter voxels. We used methodology involving operator-independent automated postprocessing and quantification of Glx and lactate levels in CNS. Brain metabolite concentrations were related to serum bilirubin concentration and other blood parameters of liver function.
Patients and methods
Patients
Nine pediatric patients (age 3 months-11 year) were examined by brain MRI/MRS as part of a workup to assess the state of the patient, either during screening for subsequent treatment (patients 1–5) or as part of checkup after successful transplantation (patient 4 second time, patients 6–9) (Table 1). MRI of the latter patients was indicated to investigate the presence of intracranial hypertension (patient 4 second time), intracranial lesions in syndrome of Alagille (patients 6, 8), structural defects as the cause of headaches (patient 7) or ischemic lesions after an epileptic episode (patient 9). Patients 2–5 were diagnosed with ALF, illustrated by a vitamin K-resistant coagulation abnormality (an international normalized ratio INR ≥1.5) [10] and any degree of mental alteration (encephalopathy) at the time of MRS. Strictly speaking, patient 1 had “acute on chronic” liver failure, with hepatic encephalopathy. Encephalopathy was determined according to Suchy [11]. Patient 4 had ALF because of a non-functioning graft after liver transplantation. The second MRS study in patient 4 and those in patients 6–9 were performed when they had neither ALF nor encephalopathy and were used as controls, considering that their MR studies were negative for all of the above-mentioned indications. This approach was based on institutional medical ethical guidelines excluding the participation of young healthy children as control group in MRI studies. Table 2 gives the values of biochemical blood parameters for biliary cholestasis (bilirubin, γ-glutamyl transpeptidase, alkaline phosphatase), hepatic detoxification (ammonia), protein synthesis (albumin) and liver parenchymal integrity (aspartate aminotransferase, alanine aminotransferase). Table 2 also provides venous pH and lactate (bottom two rows) and the brain tissue metabolite concentrations as determined by MRS in the patients (top two rows). The indirect bilirubin concentrations were derived by subtracting direct bilirubin from total bilirubin, both determined by routine biochemical analysis. Reference values for bilirubin, alkaline phoshatase and albumin were adopted from Atallah et al. [12], those for γ-glutamyltranspeptidase, aspartate aminotransferase and alanine aminotransferase from Lee at al. [13]. Reference values for venous ammonia samples, pH and blood lactate were based on historical values from our own laboratory. The MRS reference range for Glx and for lactate had been obtained from our own MRS data base of age-matched children without neurological problems. Our data cannot be matched to previously published data from other groups, because others have not differentiated between grey and white matter and/or not summarized the signals from different voxels necessary in order to be able to quantify Glx and lactate signals. All MRI/MRS examinations were performed with informed consent, and blood samples were collected on the same day. The missing values for blood lactate (patients 2, 3, 6, 7, 8) and ammonia (patients 6, 7, 8) in Table 2 reflect lack of clinically indication for having these determined on the day of MRS. Patients 1–3 did not survive ALF or liver transplantation, due to failing liver grafts and abstinence from retransplantation because of progressive circulatory insufficiency with multi-organ failure, ongoing adenovirus infection with circulatory insufficiency and/or ongoing systemic yeast infection.
MR spectroscopy
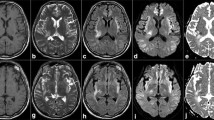
MRI and proton (1H) MRS were performed using an eight-channel transmit/receive head coil of a 1.5-T Magnetom Sonata system (Siemens AG, Erlangen, Germany). The MRI protocol consisted of transverse T2-weighted fast spin-echo, T2-weighted gradient echo, fluid attenuated inversion recovery (FLAIR), inversion recovery and diffusion-weighted imaging (DWI) covering the entire brain. Sagittal T1-weighted spin-echo and coronal T2-weighted fast spin-echo were also acquired. Two-dimensionalchemical shift imaging (CSI) point resolved spectroscopy measurement with a repetition time (TR) of 1,500 ms and an echo time (TE) of 135 ms [14]. A transverse T2-weighted fast spin-echo series was used as guidance for examining an approximately 7 × 7 × 2 cm3 supraventricular volume of interest (i.e., located cranial to the ventricles). Use of a 16 × 16 phase encoded field of view of 16 × 16 cm2 resulted in MRS voxels of 1 × 1 × 2 cm3 (7 min acquisition time). The number of peaks fitted included the chemical shift ranges restricted to 3.1–3.3 ppm for choline, 2.9–3.1 for creatine, 2.2–2.4 for glutamate/glutamine (Glx), 1.9–2.1 for N-acetyl aspartate, and 1.2–1.4 for lactate. Using a standardized postprocessing protocol, the raw data were processed automatically, allowing for operator-independent quantifications. The CSI voxels on the edge of the volume of interest (subject to signal drop-off) were deducted from the total data matrix. The inner 36 voxels were analyzed and separated into the two central columns mainly containing grey matter (12 voxels) and the remainder of 24 voxels filled with white matter (Fig. 1, right side) [15]. Grey matter and white matter patient metabolite levels were determined relative to grey matter creatine as internal reference (set at 6.2 mM) [15].
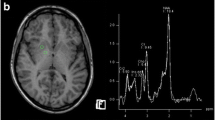
1H-MR spectra of grey matter brain tissue near the interhemispheric fissure in two patients after liver transplantation, patients 9 (a) and 3 (b) (TR/TE = 1,500/135 ms), together with the corresponding 6 × 6 spectral maps projected upon MR images showing the transverse dimensions of the volume of interest. In patient 3 (b), suffering from acute liver failure and hepatic encaphalopathy, the Glx peaks at 2.36 and 3.78 ppm are elevated and an inverted lactate doublet signal is seen at 1.33 ppm
Statistical analysis
In patients with ALF-related encephalopathy (n = 5) and in controls (n = 5), correlations between parameters for samples without normal distributions were evaluated by assessment of Spearman’s rank correlation coefficient (two-tailed). Kruskal-Wallis test was used for analyzing the asymptotic significance of differences between multiple pairs of independent samples. The two sets of divergent results in the one patient examined both before and after liver transplantation (patient 4) were both included, based on the observation that the second data set reflected a condition without ALF. This resulted in respective ALF and control group sizes of five and two for ammonia, of three and two for blood lactate and of five and five for all other parameters.
Results
The concentrations of ammonia, bilirubin, alkaline phosphatase, aspartate aminotransferase and alanine aminotransferase in blood and the MRS measured Glx and lactate levels in the brain exceeded the reference values in the five ALF patients (Table 2). Figure 1 shows normal MRS grey matter spectra of a control patient (patient 9; Fig. 1a) and high concentrations of Glx and lactate in an ALF patient (patient 3; Fig. 1b). Patient 3 also had strongly elevated ammonia and bilirubin levels (Table 2). In patient 5, the blood ammonia and CNS lactate levels were normal. The high CNS lactate concentrations in ALF patients 1 and 3 were paralleled by elevated lactate concentrations in blood (patient 1) and reduced venous pH (patients 1 and 3).
Compared with the ALF group, the concentrations of bilirubin, aspartate aminotransferase, alanine aminotransferase, albumin and of Glx measured by MRS were significantly lower in the control patients (Table 3; 0.009 ≤ P ≤ 0.046). Ammonia and CNS grey matter lactate levels were also lower in control patients, but this did not reach the level of statistical significance (P = 0.053 and 0.056, respectively; white matter lactate: P = 0.019). Figure 2 illustrates that the concentrations of Glx in the grey and white matter brain tissue and of bilirubin in blood exceeded those in the non-ALF controls, without any overlap in the respective concentration ranges (Table 2; P < 0.01, each).
Within the ALF group, grey and white matter Glx correlated inversely with venous pH (rsp = -1.00, P < 0.001, both), whereas grey and white matter lactate showed significant rank correlation with ammonia (rsp = 0.90, P = 0.037; and rsp = 0.98, P = 0.005, respectively; Fig. 3). Figure 4 illustrates the relationship between encephalopathy grades and either Glx in the grey and white matter or lactate (not significant). The levels of choline and N-acetyl aspartate showed no significant variation among the ten pediatric patients and subgroups, and were similar to those in (near) healthy children of the same age (not shown). The control Glx and lactate levels slightly exceeded/were similar to the corresponding reference levels (Glx grey matter 2.1–2.7 vs. 1.5–2.0; Glx white matter 1.2–2.3 vs. 1.0–1.5; lactate grey matter 0.3–0.6 vs. 0–0.5; lactate white matter 0.2–0.3 vs. 0.5 mmol /l) (Tables 1, 2).
Plots of brain tissue glutamate/glutamine concentration against venous pH (a) and of brain tissue lactate concentration against the ammonia concentration in blood (b). Closed symbols, acute liver failure patients; open symbols, controls; circles, grey matter brain tissue; triangles, white matter brain tissue
According to recent analyses glutamate has resonance groups centered at resonance frequencies of 2.10 ppm (β-CH2), 2.37 ppm (γ-CH2) and 3.78 ppm (α-CH) and glutamine resonates at 2.14 ppm, 2.46 ppm and 3.80 ppm [16–18]. In the spectra of Fig. 1, no peak is discernable near 2.1 ppm (probably too close to the intense N-acetyl aspartate peak), but well-defined peaks near 2.34 ppm and at 3.78 ppm are found. The position of the γ-CH2 Glx at 2.34 ppm in all ALF patients and controls indicates that the main component of the Glx peaks in the white and grey matter brain tissue is glutamate rather than glutamine.
Discussion
Our MRS study of ALF patients with encephalopathy allows for the first time the comparison in terms of specific metabolites in different patients, differentiation between grey and white matter, and finally, results that could reasonably be considered representative of the brain as a whole. We compared brain metabolism in pediatric ALF patients with various degrees of encephalopathy with a control group. ALF patients had higher Glx and lactate concentrations in brain white matter than controls (Glx + 125%: P < 0.01; lactate + 33%, P < 0.05) and higher Glx in grey matter (Glx + 125%: P < 0.01). Two patients from the control group had their MRS examination just 2 weeks after liver transplantation as compared with 1 to 5 years in the others. This may have had a negative impact on the differentiation of ALF patients from controls, as illustrated by the comparatively high bilirubin, low albumin and high blood lactate level in patient 9.
Within the group of ALF patients, positive correlations were found between grey or white matter lactate concentration and serum ammonia (P < 0.05), and negative correlations between grey or white matter Glx and venous pH (P < 0.001). The chemical shift position of the γ-CH2 resonance of Glx (near 2.34 ppm in all MR spectra) suggested that the Glx elevation in grey and white matter brain tissue of ALF patients was predominantly due to glutamate rather than to glutamine.
Using multivoxel 1H MRS can give us information about the distribution of metabolite concentrations throughout the brain. The distribution between different areas of grey and white matter would be of interest by providing information about selective vulnerability of the brain, possibly related to the degree of activity, neuronal density or other factors. This could possibly help to differentiate between different patterns of injury and prognosis. In our study we used 2D CSI, having the advantage of the acquiring of MRS measurements in 7 min and the disadvantage that only one plane of voxels is sampled without the option of retrospective positioning in the transverse direction. One would have to resort to more time-consuming 3D CSI examinations in order to assess metabolite concentrations throughout the brain, which is not an option in pediatric MRI studies. Another thought is to include the results of diffusion-weighted imaging (diffusion tensor imaging) in the evaluations of ALF patients and correlate the MRS findings with the spatial distributions of the apparent diffusion coefficients (and fractional anisotropy). That was not done in this study because some of our earliest patients were not examined by diffusion-weighted imaging.
Glutamate/glutamine (Glx)
Numerous studies have shown the accumulation in the CNS of glutamine, considered to be an indicator of the exposure of the brain to ammonia in ALF [19]. It has been proposed that patients with hepatic encephalopathy have increased cerebrospinal fluid glutamine concentrations arising from ammonia and glutamate by the action of the enzyme glutamine synthetase located in the astrocytes [5]. High glutamine concentrations have been observed in cerebrospinal fluid, serum, extracellular fluids and tissue extracts of patients with ALF [18]. Although it is conceivable that brain glutamine participates in the development of hepatic encophalopathy, glutamine per se is not neuronally active, and therefore its increase is unlikely to cause the neuroinhibitory characteristic of encephalopathy [20]. A demonstration of elevated concentrations of the neurotoxic [21] amino acid glutamate rather than glutamine in brain would therefore be of clinical relevance. Our study indeed provides strong indications of high glutamate levels in human grey and white matter tissue in ALF-related encephalopathy. In the previous study involving ALF patients (four children), McConnal et al. did not evaluate the (in their case comparatively small) peak near 2.3 ppm. Rather these scientists interpreted the presence of an intense peak at 3.75 ppm as evidence that the Glx peak represented glutamine [7]. In our data, however, the position of the γ-CH2 Glx at 2.34 ppm in all ALF patients and controls indicates that the main component of the Glx peaks in the white and grey matter brain tissue of ALF patients and controls is glutamate. Considering that sharply elevated extracellular brain glutamate concentrations have been observed in ALF, up to a concentration of 0.268 mmol/l [22], the possibility of part of the Glx signal representing extracellular rather than intracellular glutamate should be considered. We conclude that of the total of up to 6 mmol/l Glx observed in the brains of ALF patients (Table 2), up to 5% might have been extracellular glutamate.
Given the absence of sensitive parameters for encephalopathy, we determined the relationships between Glx and lactate with the traditional liver function parameters. Our observation of strong negative rank correlations between grey and white matter Glx and venous pH in patients with ALF-related encephalopathy (Fig. 3a) suggests a relationship between blood parameters and brain metabolism. This interpretation would accord with an observation by Silva et al. that the uptake of glutamate by cultered rat astrocytes is inhibited at pH values of 7.4 to 7.8 as compared with pH = 7.0 [23]. We conclude that our present data are best explained by the concept that the increased brain tissue Glx in ALF might represent increased glutamate concentrations, partly associated with pH decrease. The pH of blood, though generally associated with ALF-related lactic acidosis [24], did not significantly correlate with brain lactate concentration.
Lactate
In ALF patients the serum concentrations of ammonia correlated significantly with grey and white matter lactate concentration (Fig. 3b, closed symbols), indicating that white matter lactate as well as grey and white matter Glx could be considered as markers of cerebral consequences of ALF. The lack of differences for the most specific indicators of cholestasis (γ-glutamyl transpeptidase, alkaline phosphatase) between the ALF and control groups (Table 3) and the observed lack of correlation between the MRS determined CNS metabolites with the concentrations of γ-glutamyl transpeptidase and alkaline phosphatase in blood indicates that (chronic) cholestasis per se does not influence brain Glx and lactate levels.
The neurological impact of cholestasis
Hepatic encephalopathy has been associated with increased blood ammonia concentrations and with neurotoxicity mediated by cerebral ammonia conversion into amino acids such as glutamate and glutamine [16]. Indeed, the five patients with hepatic encephalopathy had relatively high blood ammonia concentrations, although in general ALF patients had higher CNS Glx and lactate concentrations than the controls (Table 3). There were no significant differences in CNS Glx and lactate levels between low and high grade encephalopathy patients (compare grade 1 with grades 3/4) (Fig. 4). This lack of difference may reflect the small number of patients and/or difficulty in scoring the encephalopathy grade reliably, because of sedation and/or young age.
In children with ALF CNS concentrations of Glx (grey matter, white matter) and lactate (white matter) are increased as compared with controls. Our study contrasts with a previous study of cirrhotic patients reporting hepatic encephalopathy changes in MR spectra in white matter only [8]. In the latter study the spectra in grey matter regions such as basal ganglia and frontal cortex were prone to artifacts; however, this was not the case in the present study. Here we have shown for the first time that grey and white matter Glx levels in ALF correlate inversely with venous pH, whereas grey and white matter lactate show significant rank correlation with blood ammonia level. We conclude that according to the results of MRS and the observed correlations of white and grey matter Glx and lactate concentrations with venous pH and ammonia levels, ALF is expressed in alterations in the metabolism of both grey and white matter brain tissue. Our MRS observations were paralleled by elevated serum levels of ammonia, bilirubin, aspartate aminotransferase and alanine aminotransferase. Apart from the significant correlations between CNS Glx with venous pH and of CNS lactate with blood ammonia in the patients with ALF-related encephalopathy, significant correlations of MRS parameters with any blood liver function parameter were not observed in ALF or control patients. The present results indicate that CNS Glx and lactate primarily relate to hepatic detoxification (ammonia, venous pH), rather than to liver parenchymal integrity (aspartate aminotransferase, alanine aminotransferase) or biliary cholestasis (bilirubin, γ-glutamyl transpeptidase, alkaline phosphatase).
The added value of our results for treatment and prognosis of children with ALF is limited. The brain metabolites appear to correlate better with the abnormal biochemistry than with clinical encephalopathy. One inference that might be drawn is that these metabolite changes are a direct reflection of the abnormal biochemistry, with the relationship to clinical encephalopathy remaining just as elusive as without the MRS result. This opens an opportunity for further discussion, which may be addressed by an MRS study of subjects with ALF but without encephalopathy.
Abbreviations
- ALF:
-
acute liver failure
- Glx:
-
glutamate/glutamine
- MRS:
-
magnetic resonance spectroscopy
References
Bustamante J, Rimola A, Ventura PJ, Navasa M, Cirera I, Reggiardo V, Rodes J (1999) Prognostic significance of hepatic encephalopathy in patients with cirrhosis. J Hepatol 30:890–895
Hui AY, Chan AL, Leung NW, Hung LC, Chan FK, Sung JJ (2002) Survival and prognostic indicators in patients with hepatitis B virus-related cirrhosis after onset of hepatic decompensation. J Clin Gastroenterol 34:569–572
Ranjan P, Mishtra AM, Kale R, Saraswat VA, Gupta RK (2005) Cytotoxic edema is responsible for raised intracranial pressure in fulminant hepatic failure: in vivo demonstration using diffusion-weighted MRI in human subjects. Metab Brain Dis 20:181–192
Oakden WK, Moore AM, Blaser S, Noseworthy MD (2005) Spectroscopic characteristics of kernicterus: a possible metabolic signature. Am J Neuroradiol 26:1571–1574
Kreis R, Ross BD, Farrow NA, Ackerman Z (1992) Metabolic disorders of the brain in chronic hepatic encephalopathy detected with 1H MR spectroscopy. Radiology 182:19–27
Gupta RK, Saraswat VA, Poptani H, Dhiman RK, Kohli A, Gujral RB, Naik SR (1993) Magnetic resonance imaging and localized in vivo proton spectroscopy in patients with fulminant hepatic failure. Am J Gastroenterol 88:670–674
McConnell JR, Antonson DL, Ong SS, Chu W-K, Fox IJ, Heffron TG, Langnas AN, Shaw BW Jr (1995) Proton spectroscopy of brain glutamine in acute liver failure. Hepatology 22:69–74
Weissenborn K, Björn Ahl, Fisher-Wasels D, van den Hoff J, Hecker H, Burchert W, Köstler H (2007) Correlations between magnetic resonance spectroscopy alterations and cerebral ammonia and glucose metabolism in cirrhotic patients with and without hepatic encephalopathy. Gut 56:1736–1742
Taylor-Robinson SD, Sargentoni J, Marcus CD, Morgan MY, Bryant DJ (1994) Regional variations in cerebral proton spectroscopy in patients with chronic hepatic encephalopathy. Metab Brain Dis 9:347–359
Polson J, Lee WM (2005) AASLD position paper: the management of acute liver failure. Hepatology 41:1179–1197
Suchy FJ (1996) Fulminant Hepatic failure. In: Behrman RE, Kliegman RM, Arvin AM (eds) Nelson textbook of pediatrics. WB Saunders Company. 15th edition: 1151
Atallah AM, Shiha GE, Omran MM, Zalata KR (2006) A discriminant score based on four routine laboratory blood tests for accurate diagnosis of severe fibrosis and /or liver cirrhosis in Egyptian patients with chronic hepatitis C. Hepatol Res 34:163–169
Lee S, Kim YJ, Jeon TY, Kim HH, Oh SW, Park Y, Kim SS (2006) Obesity is the only independent factor associated with ultrasound-diagnosed non-alcoholic fatty liver disease: a cross sectional case-control study. Scand J Gastroenterol 41:566–572
Sijens PE, van den Bent MJ, Nowak PJCM, van Dijk P, Oudkerk M (1997) 1H Chemical shift imaging reveals loss of brain tumor choline signal after administration of Gd-contrast agent. Magn Reson Med 37:222–225
Sijens PE, Mostert JP, Oudkerk M, De Keyser J (2006) 1H MR Spectroscopy of the brain in multiple sclerosis subtypes with analysis of the metabolite concentrations in gray and white matter: initial findings. Eur Radiol 16:489–495
Zwingman C, Butterworth RF (2005) An update on the role of brain glutamine synthesis and its relation to cell-specific energy metabolism in the hyperammonemic brain: further study using NMR spectroscopy. Neurochem Intern 47:19–30
Zwingman C, Chatauret N, Leibfritz D, Butterworth RG (2003) Selective increase of brain lactate synthesis in experimental acute liver failure: results of a [1H-13C] nuclear magnetic resonance study. Hepatology 37:420–428
Silwood CJL, Lynch EJ, Seddon S, Sheerin A, Claxson AWD, Grootveld MC (1999) 1H-NMR analysis of microbial-derived organic acids in primary root carious lesions and saliva. NMR Biomed 12:345–356
Tugnoli V, Mucci A, Schenetti L, Calabrese C, Di Febo G, Rossi MC, Tosi MR (2004) Molecular characterization of human gastric mucosa by HR-MAS magnetic resonance spectroscopy. Int J Mol Med 14:1065–1071
Cordoba J, Alonso J, Rovira A, Jacas C, Sanpedro F, Castells L, Vargas V, Margarit C, Kulisewsky J, Eteban R, Guardia J (2001) The development of low-grade cerebral edema in cirrhosis is supported by the evolution of 1H-magnetic resonance abnormalities after liver transplantation. J Hepatol 35:598–604
Schinder AF, Olson EC, Spitzer NC, Montal M (1996) Mitochondrial dysfunction is a primary event in glutamate neurotoxicity. J Neurosci 16:6125–6133
Tofteng F, Larsen FS (2004) The effect of indomethacin on intracranial pressure, cerebral prefusion and extracellular lactate and glutamate concentrations in patients with fulminant hepatic failure. J Cereb Blood Flow Metab 24:798–804
Silva R, Mata L, Gulbenkian S, Brito MA, Tiribelli C, Brites D (1999) Inhibition of glutamate uptake by unconjugated bilirubin in cultured cortical rat astrocytes; role of concentration and pH. Biochem Biophys Res Comm 265:67–72
Funk G-C, Doberer D, Fuhrmann V, Holzinger U, Kitzberger R, Kneidinger N, Lindner G, Schneweiss B (2006) The acidifying effect of lactate is neutralized by the alkalinizing effect of hypoalbuminemia in non-paracetamol-induced acute liver failure. J Hepatol 45:387–392
Acknowledgement
We thank Dr. D.A. Sival from Pediatrics, Beatrix Children’s Hospital UMCG, for helpful discussions.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Sijens, P.E., Alkefaji, H., Lunsing, R.J. et al. Quantitative multivoxel 1H MR spectroscopy of the brain in children with acute liver failure. Eur Radiol 18, 2601–2609 (2008). https://doi.org/10.1007/s00330-008-1049-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-008-1049-z