Abstract
Objectives
This prenatal MRI study evaluated the potential of diffusion tensor imaging (DTI) metrics to identify changes in the midbrain of fetuses with Chiari II malformations compared to fetuses with mild ventriculomegaly, hydrocephalus and normal CNS development.
Methods
Fractional anisotropy (FA) and apparent diffusion coefficient (ADC) were calculated from a region of interest (ROI) in the midbrain of 46 fetuses with normal CNS, 15 with Chiari II malformations, eight with hydrocephalus and 12 with mild ventriculomegaly. Fetuses with different diagnoses were compared group-wise after age-matching. Axial T2W-FSE sequences and single-shot echo planar DTI sequences (16 non-collinear diffusion gradient-encoding directions, b-values of 0 and 700 s/mm2, 1.5 Tesla) were evaluated retrospectively.
Results
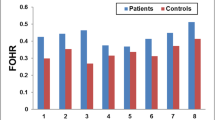
In Chiari II malformations, FA was significantly higher than in age-matched fetuses with a normal CNS (p = .003), while ADC was not significantly different. No differences in DTI metrics between normal controls and fetuses with hydrocephalus or vetriculomegaly were detected.
Conclusions
DTI can detect and quantify parenchymal alterations of the fetal midbrain in Chiari II malformations. Therefore, in cases of enlarged fetal ventricles, FA of the fetal midbrain may contribute to the differentiation between Chiari II malformation and other entities.
Key Points
• FA in the fetal midbrain is elevated in Chiari II malformations.
• FA is not elevated in hydrocephalus and mild ventriculomegaly without Chiari II.
• Measuring FA may help distinguish different causes for enlarged ventricles prenatally.
• Elevated FA may aid in the diagnosis of open neural tube defects.
• Elevated FA might contribute to stratification for prenatal surgery in Chiari II.



Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- CNS:
-
Central nervous system
- DTI:
-
Diffusion tensor imaging
- FA:
-
Fractional anisotropy
- GW:
-
Gestational weeks/ weeks of gestation
- ROI(s):
-
Region(s) of interest
- SSFSE:
-
Single-shot fast spin-echo
- TE:
-
Echo time
- TR:
-
Repetition time
References
Kahn L, Mbabuike N, Valle-Giler EP et al (2014) Fetal surgery: the ochsner experience with in utero spina bifida repair. Ochsner J 14:112–118
Lorber J, Ward AM (1985) Spina bifida--a vanishing nightmare? Arch Dis Child 60:1086–1091
Milunsky A, Jick SS, Bruell CL et al (1989) Predictive values, relative risks, and overall benefits of high and low maternal serum alpha-fetoprotein screening in singleton pregnancies: new epidemiologic data. Am J Obstet Gynecol 161:291–297
Chaoui R, Benoit B, Mitkowska-Wozniak H, Heling KS, Nicolaides KH (2009) Assessment of intracranial translucency (IT) in the detection of spina bifida at the 11-13-week scan. Ultrasound Obstet Gynecol 34:249–252
Nishino A, Shirane R, So K, Arai H, Suzuki H, Sakurai Y (1998) Cervical myelocystocele with Chiari II malformation: magnetic resonance imaging and surgical treatment. Surg Neurol 49:269–273
Tortori-Donati P, Rossi AMD, Biancheri R (2005) Pediatric neuroradiology. Springer, Berlin [Great Britain]
Stevenson KL (2004) Chiari Type II malformation: past, present, and future. Neurosurg Focus 16, E5
Sutton LN, Adzick NS, Bilaniuk LT, Johnson MP, Crombleholme TM, Flake AW (1999) Improvement in hindbrain herniation demonstrated by serial fetal magnetic resonance imaging following fetal surgery for myelomeningocele. JAMA 282:1826–1831
Sutton LN (2008) Fetal surgery for neural tube defects. Best Pract Res Clin Obstet Gynaecol 22:175–188
Adzick NS, Thom EA, Spong CY et al (2011) A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med 364:993–1004
McLone DG, Knepper PA (1989) The cause of Chiari II malformation: a unified theory. Pediatr Neurosci 15:1–12
McLone DG, Dias MS (2003) The Chiari II malformation: cause and impact. Childs Nerv Syst 19:540–550
Sarnat HB (2008) Disorders of segmentation of the neural tube: Chiari malformations. Handb Clin Neurol 87:89–103
Sarnat HB (2004) Regional ependymal upregulation of vimentin in Chiari II malformation, aqueductal stenosis, and hydromyelia. Pediatr Dev Pathol 7:48–60
Safra N, Bassuk AG, Ferguson PJ et al (2013) Genome-wide association mapping in dogs enables identification of the homeobox gene, NKX2-8, as a genetic component of neural tube defects in humans. PLoS Genet 9, e1003646
Pollack IF, Pang D, Kocoshis S, Putnam P (1992) Neurogenic dysphagia resulting from Chiari malformations. Neurosurgery 30:709–719
Alsaadi MM, Iqbal SM, Elgamal EA, Gozal D (2012) Sleep-disordered breathing in children with Chiari malformation type II and myelomeningocele. Pediatr Int 54:623–626
Sieben RL, Hamida MB, Shulman K (1971) Multiple cranial nerve deficits associated with the Arnold-Chiari malformation. Neurology 21:673–681
Ocal E, Irwin B, Cochrane D, Singhal A, Steinbok P (2012) Stridor at birth predicts poor outcome in neonates with myelomeningocele. Childs Nerv Syst 28:265–271
Gilbert JN, Jones KL, Rorke LB, Chernoff GF, James HE (1986) Central nervous system anomalies associated with meningomyelocele, hydrocephalus, and the Arnold-Chiari malformation: reappraisal of theories regarding the pathogenesis of posterior neural tube closure defects. Neurosurgery 18:559–564
Boyd PA, Devigan C, Khoshnood B, Loane M, Garne E, Dolk H (2008) Survey of prenatal screening policies in Europe for structural malformations and chromosome anomalies, and their impact on detection and termination rates for neural tube defects and Down's syndrome. BJOG 115:689–696
Van den Hof MC, Nicolaides KH, Campbell J, Campbell S (1990) Evaluation of the lemon and banana signs in one hundred thirty fetuses with open spina bifida. Am J Obstet Gynecol 162:322–327
Campbell J, Gilbert WM, Nicolaides KH, Campbell S (1987) Ultrasound screening for spina bifida: cranial and cerebellar signs in a high-risk population. Obstet Gynecol 70:247–250
Thomas M (2003) The lemon sign. Radiology 228:206–207
D'Addario V, Pinto V, Del Bianco A et al (2001) The clivus-supraocciput angle: a useful measurement to evaluate the shape and size of the fetal posterior fossa and to diagnose Chiari II malformation. Ultrasound Obstet Gynecol 18:146–149
D'Addario V, Rossi AC, Pinto V, Pintucci A, Di Cagno L (2008) Comparison of six sonographic signs in the prenatal diagnosis of spina bifida. J Perinat Med 36:330–334
Woitek R, Dvorak A, Weber M et al (2014) MR-based morphometry of the posterior fossa in fetuses with neural tube defects of the spine. PLoS One 9, e112585
Chen SC, Simon EM, Haselgrove JC et al (2006) Fetal posterior fossa volume: assessment with MR imaging. Radiology 238:997–1003
Lachmann R, Chaoui R, Moratalla J, Picciarelli G, Nicolaides KH (2011) Posterior brain in fetuses with open spina bifida at 11 to 13 weeks. Prenat Diagn 31:103–106
Alonso A, Hernan MA (2008) Temporal trends in the incidence of multiple sclerosis: a systematic review. Neurology 71:129–135
Chang Y, Jung TD, Yoo DS, Hyun JK (2010) Diffusion tensor imaging and fiber tractography of patients with cervical spinal cord injury. J Neurotrauma 27:2033–2040
Sakai T, Miyagi R, Yamabe E, Fujinaga Y, Bhatia NN, Yoshioka H (2014) Diffusion-weighted imaging and diffusion tensor imaging of asymptomatic lumbar disc herniation. J Med Investig 61:197–203
Eguchi Y, Ohtori S, Orita S et al (2011) Quantitative evaluation and visualization of lumbar foraminal nerve root entrapment by using diffusion tensor imaging: preliminary results. AJNR Am J Neuroradiol 32:1824–1829
Balbi V, Budzik JF, Duhamel A, Bera-Louville A, Le Thuc V, Cotten A (2011) Tractography of lumbar nerve roots: initial results. Eur Radiol 21:1153–1159
Jengojan S, Kovar F, Breitenseher J, Weber M, Prayer D, Kasprian G (2015) Acute radial nerve entrapment at the spiral groove: detection by DTI-based neurography. Eur Radiol. doi:10.1007/s00330-014-3562-6
Eshetu T, Meoded A, Jallo GI, Carson BS, Huisman TA, Poretti A (2014) Diffusion tensor imaging in pediatric Chiari type I malformation. Dev Med Child Neurol 56:742–748
Kasprian G, Brugger PC, Weber M et al (2008) In utero tractography of fetal white matter development. Neuroimage 43:213–224
Griffiths PD, Reeves MJ, Morris JE et al (2010) A prospective study of fetuses with isolated ventriculomegaly investigated by antenatal sonography and in utero MR imaging. AJNR Am J Neuroradiol 31:106–111
Watanabe M, Jo J, Radu A, Kaneko M, Tabata Y, Flake AW (2010) A tissue engineering approach for prenatal closure of myelomeningocele with gelatin sponges incorporating basic fibroblast growth factor. Tissue Eng A 16:1645–1655
Watanabe M, Li H, Roybal J et al (2011) A tissue engineering approach for prenatal closure of myelomeningocele: comparison of gelatin sponge and microsphere scaffolds and bioactive protein coatings. Tissue Eng A 17:1099–1110
Watanabe M, Kim AG, Flake AW (2014) Tissue Engineering Strategies for Fetal Myelomeningocele Repair in Animal Models. Fetal Diagn Ther. doi:10.1159/000362931
Kohl T, Tchatcheva K, Merz W et al (2009) Percutaneous fetoscopic patch closure of human spina bifida aperta: advances in fetal surgical techniques may obviate the need for early postnatal neurosurgical intervention. Surg Endosc 23:890–895
Verbeek RJ, Heep A, Maurits NM et al (2012) Fetal endoscopic myelomeningocele closure preserves segmental neurological function. Dev Med Child Neurol 54:15–22
Kasprian G, Amann G, Panotopoulos J et al (2014) Peripheral nerve tractography in soft tissue tumors: A preliminary 3-tesla diffusion tensor magnetic resonance imaging study. Muscle Nerve. doi:10.1002/mus.24313
Mukherjee P, Miller JH, Shimony JS et al (2002) Diffusion-tensor MR imaging of gray and white matter development during normal human brain maturation. AJNR Am J Neuroradiol 23:1445–1456
Boujraf S, Luypaert R, Shabana W, De Meirleir L, Sourbron S, Osteaux M (2002) Study of pediatric brain development using magnetic resonance imaging of anisotropic diffusion. Magn Reson Imaging 20:327–336
Huppi PS, Dubois J (2006) Diffusion tensor imaging of brain development. Semin Fetal Neonatal Med 11:489–497
Madler B, Drabycz SA, Kolind SH, Whittall KP, MacKay AL (2008) Is diffusion anisotropy an accurate monitor of myelination? Correlation of multicomponent T2 relaxation and diffusion tensor anisotropy in human brain. Magn Reson Imaging 26:874–888
Wimberger DM, Roberts TP, Barkovich AJ, Prayer LM, Moseley ME, Kucharczyk J (1995) Identification of “premyelination” by diffusion-weighted MRI. J Comput Assist Tomogr 19:28–33
Prayer D, Barkovich AJ, Kirschner DA et al (2001) Visualization of nonstructural changes in early white matter development on diffusion-weighted MR images: evidence supporting premyelination anisotropy. AJNR Am J Neuroradiol 22:1572–1576
Mitter C, Kasprian G, Brugger PC, Prayer D (2011) Three-dimensional visualization of fetal white-matter pathways in utero. Ultrasound Obstet Gynecol 37:252–253
Kasprian G, Brugger PC, Schopf V et al (2013) Assessing prenatal white matter connectivity in commissural agenesis. Brain 136:168–179
Acknowledgments
The scientific guarantor of this publication is Gregor Kasprian, MD (Senior Author). The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding.
Michael Weber (Co-Author) kindly provided statistical advice for this manuscript and has significant statistical expertise. Institutional Review Board approval was obtained. Written informed consent was waived by the Institutional Review Board. Some study subjects or cohorts have been previously reported in Woitek R, Dvorak A, Weber M, et al (2014) MR-based morphometry of the posterior fossa in fetuses with neural tube defects of the spine. PLoS One 9:e112585
Methodology: retrospective, diagnostic study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Woitek, R., Prayer, D., Weber, M. et al. Fetal diffusion tensor quantification of brainstem pathology in Chiari II malformation. Eur Radiol 26, 1274–1283 (2016). https://doi.org/10.1007/s00330-015-3939-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-3939-1