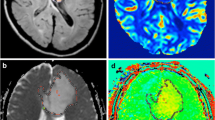
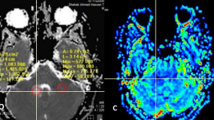
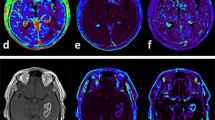
Abstract
Objectives
To determine the added value of amide proton transfer (APT) imaging to conventional and perfusion MRI for differentiating tumour progression (TP) from the treatment-related effect (TE) in patients with post-treatment glioblastomas.
Methods
Sixty-five consecutive patients with enlarging contrast-enhancing lesions following concurrent chemoradiotherapy were assessed using contrast-enhanced T1-weighted MRI (CE-T1WI), 90th percentile histogram parameters of normalized cerebral blood volume (nCBV90) and APT asymmetry value (APT90). Diagnostic performance was determined using the area under the receiver operating characteristic curve (AUC) and cross validations.
Results
There were statistically significant differences in the mean APT90 between the TP and the TE groups (3.87–4.01 % vs. 1.38–1.41 %; P < .001). Compared with CE-T1WI alone, the addition of APT90 to CE-T1WI significantly improved cross-validated AUC from 0.58–0.74 to 0.89–0.91 for differentiating TP from TE. The combination of CE-T1WI, nCBV90 and APT90 resulted in greater diagnostic accuracy for differentiating TP from TE than the combination of CE-T1WI and nCBV90 (cross-validated AUC, 0.95–0.97 vs. 0.84–0.91). The inter-reader agreement between the expert and trainee was excellent for the measurements of APT90 (intraclass correlation coefficient, 0.94).
Conclusion
Adding APT imaging to conventional and perfusion MRI improves the diagnostic performance for differentiating TP from TE.
Key Points
• APT imaging could provide a reliable distinction between TP and TE
• Adding APT imaging to CE-T1WI improved the diagnostic accuracy versus CE-T1WI alone
• Multimodal imaging using CE-T1WI, perfusion and APT imaging led to accurate diagnosis
• The inter-reader agreement of APT histogram parameters was excellent







Similar content being viewed by others
Abbreviations
- APT:
-
Amide proton transfer
- APT90:
-
90th percentile value of histogram cutoff for the amide proton transfer imaging
- AUC:
-
Area under the ROC curve
- CCRT:
-
Concurrent chemotherapy and radiation therapy
- CE-T1WI:
-
Contrast-enhanced T1 weighted image
- DSC:
-
T2*-weighted dynamic susceptibility contrast-enhanced
- DWI:
-
Diffusion-weighted imaging
- nCBV:
-
Normalized cerebral blood volume
- nCBV90:
-
90th percentile value of histogram cutoff for the normalized cerebral blood volume
- ROC:
-
Receiver operating characteristics
- ROI:
-
Region of interest
- TE:
-
Treatment-related effect
- TP:
-
Tumour progression
References
Fiveash JB, Spencer SA (2003) Role of radiation therapy and radiosurgery in glioblastoma multiforme. Cancer J 9:222–229
Mullins ME, Barest GD, Schaefer PW, Hochberg FH, Gonzalez RG, Lev MH (2005) Radiation necrosis versus glioma recurrence: conventional MR imaging clues to diagnosis. AJNR Am J Neuroradiol 26:1967–1972
Chung WJ, Kim HS, Kim N, Choi CG, Kim SJ (2013) Recurrent glioblastoma: optimum area under the curve method derived from dynamic contrast-enhanced T1-weighted perfusion MR imaging. Radiology 269:561–568
Fatterpekar GM, Galheigo D, Narayana A, Johnson G, Knopp E (2012) Treatment-related change versus tumor recurrence in high-grade gliomas: a diagnostic conundrum--use of dynamic susceptibility contrast-enhanced (DSC) perfusion MRI. AJR Am J Roentgenol 198:19–26
Kim HS, Kim JH, Kim SH, Cho KG, Kim SY (2010) Posttreatment high-grade glioma: usefulness of peak height position with semiquantitative MR perfusion histogram analysis in an entire contrast-enhanced lesion for predicting volume fraction of recurrence. Radiology 256:906–915
Kumar AJ, Leeds NE, Fuller GN et al (2000) Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology 217:377–384
Barajas RF Jr, Chang JS, Segal MR et al (2009) Differentiation of recurrent glioblastoma multiforme from radiation necrosis after external beam radiation therapy with dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology 253:486–496
Maia AC Jr, Malheiros SM, da Rocha AJ et al (2005) MR cerebral blood volume maps correlated with vascular endothelial growth factor expression and tumor grade in nonenhancing gliomas. AJNR Am J Neuroradiol 26:777–783
Sadeghi N, D'Haene N, Decaestecker C et al (2008) Apparent diffusion coefficient and cerebral blood volume in brain gliomas: relation to tumor cell density and tumor microvessel density based on stereotactic biopsies. AJNR Am J Neuroradiol 29:476–482
Wen Z, Hu S, Huang F et al (2010) MR imaging of high-grade brain tumors using endogenous protein and peptide-based contrast. NeuroImage 51:616–622
Zhou J (2011) Amide proton transfer imaging of the human brain. Methods Mol Biol 711:227–237
Zhou J, Blakeley JO, Hua J et al (2008) Practical data acquisition method for human brain tumor amide proton transfer (APT) imaging. Magn Reson Med 60:842–849
Jones CK, Schlosser MJ, van Zijl PC, Pomper MG, Golay X, Zhou J (2006) Amide proton transfer imaging of human brain tumors at 3T. Magn Reson Med 56:585–592
Sagiyama K, Mashimo T, Togao O et al (2014) In vivo chemical exchange saturation transfer imaging allows early detection of a therapeutic response in glioblastoma. Proc Natl Acad Sci U S A 111:4542–4547
Park JE, Kim HS, Park KJ, Choi CG, Kim SJ (2015) Histogram analysis of amide proton transfer imaging to identify contrast-enhancing low-grade brain tumor that mimics high-grade tumor: increased accuracy of MR perfusion. Radiology 277:151–161
Togao O, Yoshiura T, Keupp J et al (2014) Amide proton transfer imaging of adult diffuse gliomas: correlation with histopathological grades. Neuro-Oncology 16:441–448
Zhou J, Zhu H, Lim M et al (2013) Three-dimensional amide proton transfer MR imaging of gliomas: initial experience and comparison with gadolinium enhancement. J Magn Reson Imaging 38:1119–1128
Zhou J, Tryggestad E, Wen Z et al (2011) Differentiation between glioma and radiation necrosis using molecular magnetic resonance imaging of endogenous proteins and peptides. Nat Med 17:130–134
Bastin ME, Carpenter TK, Armitage PA, Sinha S, Wardlaw JM, Whittle IR (2006) Effects of dexamethasone on cerebral perfusion and water diffusion in patients with high-grade glioma. AJNR Am J Neuroradiol 27:402–408
Wen PY, Macdonald DR, Reardon DA et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972
Young RJ, Gupta A, Shah AD et al (2011) Potential utility of conventional MRI signs in diagnosing pseudoprogression in glioblastoma. Neurology 76:1918–1924
Moffat BA, Chenevert TL, Lawrence TS et al (2005) Functional diffusion map: a noninvasive MRI biomarker for early stratification of clinical brain tumor response. Proc Natl Acad Sci U S A 102:5524–5529
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
Xie Q, Mittal S, Berens ME (2014) Targeting adaptive glioblastoma: an overview of proliferation and invasion. Neuro-Oncology 16:1575–1584
Zhou J, Payen JF, Wilson DA, Traystman RJ, van Zijl PC (2003) Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat Med 9:1085–1090
Scheidegger R, Wong ET, Alsop DC (2014) Contributors to contrast between glioma and brain tissue in chemical exchange saturation transfer sensitive imaging at 3 Tesla. NeuroImage 99:256–268
Xu J, Zaiss M, Zu Z et al (2014) On the origins of chemical exchange saturation transfer (CEST) contrast in tumors at 9.4 T. NMR Biomed 27:406–416
Hong X, Liu L, Wang M et al (2014) Quantitative multiparametric MRI assessment of glioma response to radiotherapy in a rat model. Neuro-Oncology 16:856–867
Park JE, Kim HS, Park KJ, Kim SJ, Kim JH, Smith SA (2015) Pre- and posttreatment glioma: comparison of amide proton transfer imaging with MR spectroscopy for biomarkers of tumor proliferation. Radiology 142979
Acknowledgments
The scientific guarantor of this publication is Prof. Sang Joon Kim. This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (grant number: NRF-2014R1A2A2A01004937). The Institutional Review Board approved our human study (The Institutional Review Board of Asan Medical Center [http://eirb.amc.seoul.kr]: S2015-0578-0001). Written informed consent was waived by the Institutional Review Board. Methodology: retrospective, diagnostic or prognostic study, performed at one institution. The authors thank the Biomedical Imaging Infrastructure, Department of Radiology, Asan Medical Center for technical support in image processing, Ha Kyu Jung for technical support and valuable contributions, and Prof. Hwa Jung Kim for statistical support and valuable contributions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, K.J., Kim, H.S., Park, J.E. et al. Added value of amide proton transfer imaging to conventional and perfusion MR imaging for evaluating the treatment response of newly diagnosed glioblastoma. Eur Radiol 26, 4390–4403 (2016). https://doi.org/10.1007/s00330-016-4261-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-016-4261-2