Abstract
Objectives
To evaluate the ability of the initial area under the curve (IAUC) derived from dynamic contrast-enhanced MR imaging (DCE-MRI) and apparent diffusion coefficient (ADC) in differentiating between primary central nervous system lymphoma (PCNSL) and atypical glioblastoma (GBM).
Methods
We retrospectively identified 19 patients with atypical GBM (less than 13 % necrosis of the enhancing tumour), and 23 patients with PCNSL. The histogram parameters of IAUC at 30, 60, 90 s (IAUC30, IAUC60, and IAUC90), and ADC were compared between PCNSL and GBM. The diagnostic performances and added values of the IAUC and ADC for differentiating between PCNSL and GBM were evaluated. Interobserver agreement was assessed via intraclass correlation coefficient (ICC).
Results
The IAUC and ADC parameters were higher in GBM than in PCNSL. The 90th percentile (p90) of IAUC30 and 10th percentile (p10) of ADC showed the best diagnostic performance. Adding p90 of IAUC30 to p10 of ADC improved the differentiation between PCNSL and GBM (area under the ROC curve [AUC] = 0.886), compared to IAUC30 or ADC alone (AUC = 0.789 and 0.744; P < 0.05 for all). The ICC was 0.96 for p90 of IAUC30.
Conclusions
The IAUC may be a useful parameter together with ADC for differentiating between PCNSL and atypical GBM.
Key Points
• High reproducibility is essential for practical implementation of advanced MRI parameters.
• IAUC and ADC are highly reproducible parameters.
• IAUC values were higher in atypical GBM than in PCNSL.
• Adding IAUC to ADC improved the differentiation between PCNSL and GBM.
• IAUC with ADC are useful for differentiating PCNSL from GBM.
Similar content being viewed by others
Introduction
The differentiation between primary central nervous system lymphoma (PCNSL) and glioblastoma (GBM) is of great clinical relevance because GBM is treated with surgical resection followed by concurrent chemoradiation with temozolomide, whereas PCNSL is treated with chemotherapy with methotrexate [1, 2]. GBM and PCNSL can be differentiated in many cases based on different conventional MR images, since necrosis is commonly seen in GBM and homogeneous enhancement without necrosis favours PCNSL [3]. However, conventional MRI findings can overlap in cases of atypical GBM with no or little necrosis and atypical PCNLS with necrosis, which complicates the discrimination of GBM from PCNLS [4]. Advanced MR techniques such as diffusion tensor imaging (DTI), dynamic susceptibility contrast-enhanced (DSC), and dynamic contrast-enhanced (DCE) MRI have been investigated as adjunct imaging biomarkers for differentiating PCNSL from GBM. For instance, previous studies have reported lower relative cerebral blood volume (rCBV), higher vascular permeability (K trans), and lower apparent diffusion coefficient (ADC) values in PCNSL than in GBM [5–8]. However, most of these studies enrolled all patients with GBM and PCNLS, regardless of their findings on conventional MRI. In addition, rCBV measurement has been found to have reproducibility issues [9–12], and the measurement of the model-based parameters such as K trans has several obstacles including complicated multi-compartment physiologic models, the arterial input function, and model fit instability [13–18]. The initial area under the time to signal intensity curve (IAUC), which is obtained from DCE-MRI, is a model-free parameter that is highly reproducible and reflects multiple characters of the tumour microenvironment such as vascular permeability and perfusion [13, 19]. The IAUC has been successfully used in previous studies of various diseases including prostate, breast, cervical, and pancreatic cancers, as well as brain tumours [13, 19–24]. We hypothesized that the IAUC would be clinically helpful in the differentiation of PCNSL from atypical GBM that are indistinguishable based on conventional MRI. Thus, we chose two parameters that do not require any user-dependent input and that are expected to be highly reproducible, IAUC and ADC. We focused our study on PCNSL vs. atypical GBM instead of atypical PCNLS vs. GBM, as the incidence rate is higher for GBM than for PCNSL [25]. Moreover, atypical appearances of PCNSL in immunocompetent patients are predominant among those with Epstein-Barr virus positivity [26], and the incidence of Epstein-Barr virus associated PCNSL has been reported to be only 4.9 - 13.6 % of immunocompetent patients with PCNSL [27–29].
The purpose of this study was to assess the diagnostic ability of IAUC derived from DCE-MRI and ADC to differentiate between PCNSL and atypical GBM. This study also aimed to assess the added value of IAUC to ADC.
Materials and methods
This retrospective study was approved by our institutional review board, which waived the requirement for informed consent.
Participants
A total of 127 patients with GBMs who underwent preoperative DTI and DCE-MRI from December 2009 to July 2014 were retrospectively reviewed to select patients with atypical GBM. The inclusion criteria were: 1) pathologically confirmed GBM, 2) no prior biopsy or treatment, and 3) preoperative DCE-MRI and DTI with a b value = 600 s/mm2. Atypical GBM was defined as a GBM with less than 13 % necrosis of the enhancing tumour, since 13 % necrosis was the optimal cutoff value to discriminate between PCNSL and entire GBM of our cohort, as mentioned later. All cases of atypical GBM were determined by semiautomatic quantitative measurement of necrosis and enhancing volume on conventional MRI. Out of the 127 GBMs, 23 atypical GBMs were identified. To these, the following exclusion criteria were applied: 1) previous brain biopsy prior to MRI (n = 2), and 2) different DTI protocol with a b value = 1000 s/mm2 (n = 2).
The 27 immunocompetent patients with pathologically confirmed PCNSL who underwent pretreatment DTI and DCE-MRI were reviewed, leading to the exclusion of three patients due to previous biopsy or treatment prior to MRI, and one patient due to an inappropriate DTI protocol with a b value = 1000. Thus, 42 patients (23 with PCNSL and 19 with atypical GBM) were enrolled. The mean patient age was 59.7 ± 2.1 years and the male to female ratio was 18:24.
Imaging
A 3.0 T MRI (Achieva, Philips) and an 8-channel SENSE head coil were used for all preoperative MRI scans. The preoperative MRI protocol included the acquisition of T1-weighted (TR 2000 ms, TE 10 ms, FOV 240 mm, slice thickness 5 mm, and 256 × 256 matrix), T2-weighted (TR 3000 ms, TE 80 ms, FOV 240 mm, slice thickness 5 mm, and 256 × 256 matrix), and fluid-attenuated inversion recovery (FLAIR) images (TR 10,000 ms, TE 125 ms, FOV 240 mm, slice thickness 5 mm, and 256 × 256 matrix). 3D postcontrast T1-weighted images (TR 6.3 ms, TE 3.1 ms, FOV 240 mm, slice thickness 1 mm, and 192 x 192 matrix) were acquired after injecting gadolinium-based contrast (0.1 mL/kg gadobutrol, Gadovist, Bayer Schering Pharma).
For DCE-MRI, precontrast 3D T1-weighted images were obtained using the following parameters: TR 6.3 ms, TE 3.1 ms, FOV 240 mm, matrix 192 × 192 mm, slice thickness 3 mm, and flip angle 5°. Sixty dynamic phases of DCE T1-weighted images were obtained after precontrast scans acquired with the same MR parameters, except that a flip angle of 15° was used. After the fifth dynamic phase, gadolinium-based contrast (0.1 mL/kg gadobutrol, Gadovist, Bayer Schering Pharma) was injected at a rate of 3 mL/s. The total acquisition time for DCE-MRI was 6 min and 18 s, with each phase lasting approximately 6 s. DTI was performed with b values of 600 s/mm2 and 0 s/mm2, 32 directions, and the following parameters: TR 8413.4 ms, TE 77 ms, FOV 220 mm, slice thickness 2 mm, and 112 x 112 matrix.
Image processing
Regions of interest (ROIs) were drawn by a single neuroradiologist (Y.S.C.), to segment the entire enhancing tumour and also to avoid necrotic or cystic components using semiautomatic thresholds of signal intensity on postcontrast T1-weighted images. For quantitative measurement of necrosis proportion, necrotic or cystic components were separately segmented using the same semiautomatic thresholds of signal intensity. For IAUC values, ROIs were coregistered on DCE-MRI source images, and these coregistered ROIs were generated as separate images. The IAUC values at 30 (IAUC30), 60 (IAUC60), and 90 (IAUC90) s after the wash-in phase were calculated for each pixel, based on the previous report that used IAUC values in patients with GBM [13, 19]. Since we attempted to minimize user-dependent input, the wash-in phase was determined automatically on each pixel. The wash-in phase was defined as the initial phase showing more than a 5 % signal increase of the maximum signal increase on more than two serial phases. IAUC values were normalized to the maximum signal increase of the entire phase and outliers were removed. Next, the mean and 90th percentile (p90) of the IAUC30, IAUC60, and IAUC90 were extracted from the entire enhancing tumour. For ADC values, ROIs were coregistered on ADC maps. Then, the mean and 10th percentile (p10) of the ADC values were extracted from the entire enhancing tumour. Image analysis was conducted with the Medical Image Processing, Analysis, and Visualization software package (MIPAV, version 7.0; National Institutes of Health), and R for Windows, version 3.0.2 (R Foundation for Statistical Computing, Nashville, TN, USA).
Statistical analysis
To determine the necrosis criteria for atypical GBM, receiver operating characteristic (ROC) analysis was performed with necrosis proportion of 127 entire GBM and 27 PCNSL. The optimal cutoff necrosis proportion in the discrimination between PCNSL and GBM was calculated according to Youden’s index, which was 13 %. Thus, we defined the atypical GBM as a GBM with less than 13 % necrosis of the enhancing tumour.
The mean and p90 of IAUC30, IAUC60, and IAUC90, in addition to the mean and p10 of ADC, were compared between the PCNSL and GBM groups using Student’s t-test, according to the results of the Shapiro-Wilk test for normal distribution. For each parameter that showed a significant difference, ROC analyses were performed, and the areas under the ROC curve (AUCs) and the optimal cutoff values according to Youden’s index were calculated. In addition, the AUC after leave-one-out cross-validation was calculated to prevent overfitting. By using this method, the entire sample set except for one sample was used as a training set to build the classifier, and this trained classifier was applied to the testing sample that was not included among the training samples. This procedure was repeated until every sample was tested. Using the IAUC and ADC parameters with the highest AUC values, a multivariate diagnostic model was built. The diagnostic value of the multivariate model was compared with the values of the models using either the IAUC or the ADC alone to investigate the added values of the IAUC and ADC to each other. Considering that ROC analyses have drawbacks that necessitate relatively large odds ratios for even a small improvement, the added values of the IAUC and ADC were assessed by reclassification improvement, such as integrated discrimination improvement (IDI) and the net reclassification index (NRI) by Pencina et al. [30, 31]. The NRI assesses correctness of reclassification into different prespecified categories. The IDI is a continuous measure and is calculated as follows: integrated discrimination improvement = (ISnew – ISold) – (IPnew – IPold), where “new” refers to a model containing a new diagnostic tool of interest in addition to conventional risk predictors and “old” pertains to the model containing only the conventional predictors. IS and IP are the integrals of sensitivity and (1 – specificity), respectively. NRI and IDI values above zero indicate improved discrimination with the addition of a new parameter. Interrater reliability was assessed by calculating the intraclass coefficient (ICC) for IAUC, and ADC. All statistical analyses were performed using R for Windows, version 3.0.2 (R Foundation for Statistical Computing) and the Stata software package (version 14.0; StataCorp, College Station, TX). A P value <0.05 was considered statistically significant.
Results
Among the 19 atypical GBMs, 12 did not show any necrosis, and seven showed less than 13 % necrosis of the enhancing tumour volume, which was 6.0 ± 3.5 %. Among the 23 PCNSLs, four showed focal necrosis, which was 3.8 ± 2.5 % of the enhancing tumour volume. The mean patient age was not different between the PCNSL and GBM groups (mean ± standard deviation = 61.4 ± 13.0 years for PCNSL vs. 57.7 ± 15.0 years for GBM; P = 0.398). Similarly, the male to female ratios were not significantly different (11:12 for PCNSL vs. 7:12 for GBM; P = 0.474).
Differences in IAUC and ADC parameters between PCNSL and atypical GBM
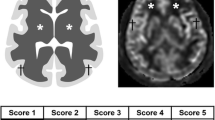
The differences in the IAUC and ADC values between PCNSL and GBM are summarized in Table 1. The IAUC values tended to be distributed in higher values in GBM than in PCNSL (Fig. 1); this tendency was statistically significant for mean of IAUC30 and p90 of IAUC30, IAUC60, and IAUC90. Representative cases of IAUC30 parameter maps of PCNSL and atypical GBM are presented in Figs. 2 and 3. The ADC values tended to be distributed in lower values in PCNSL than in GBM; this trend was statistically significant for both the mean and p10 of ADC.
Diagnostic performance, added value, and reproducibility of the IAUC and ADC
The diagnostic performances of the IAUC30, IAUC60, IAUC90, and ADC in differentiating between PCNSL and GBM are summarized in Table 2. ROC analyses revealed that the parameters with the best diagnostic performance were p90 of IAUC30 among the IAUC parameters and p10 of ADC among the ADC parameters (AUC = 0.789 and 0.744, respectively). By combining p90 of IAUC30 with p10 of ADC, the diagnostic performance was improved to an AUC of 0.886, compared to that of p10 of ADC alone (AUC = 0.744) and p90 of IAUC30 (AUC = 0.789; Table 3 and Fig. 4). This improvement was statistically significant when compared to those of p90 of IAUC (P < 0.001 by IDI and NRI) and p10 of ADC alone (P = 0.002 by IDI and NRI). The AUC value after leave-one-out cross-validation of the combination of p90 of IAUC and p10 of ADC was 0.833. Interrater reliability revealed almost perfect reproducibility for p90 of IAUC30 and p10 of ADC, with intraclass correlation coefficients of 0.96 (95 % CI: 0.91, 0.98) and 0.98 (95 % CI: 0.95, 0.99), respectively.
Discussion
We found that the IAUC values were significantly higher in GBM than in PCNSL. Moreover, the IAUC values showed fair diagnostic performance, improved diagnostic value when used in combination with ADC for discriminating PCNSL from atypical GBM, and high reproducibility. Our results imply that IAUC, in combination with ADC, may be a useful imaging biomarker for discriminating PCNSL from atypical GBM when conventional MRI findings overlap.
Many previous studies have been conducted to investigate the ability of advanced MRI techniques to differentiate PCNSL from GBM. PCNSL has been reported to be associated with lower rCBV and lower ADC values than GBM; the high cellularity of PCNSL and active angiogenesis of GBM have been proposed as possible explanations for these differences [5–8]. One recent study reported that PCNSL was associated with higher K trans values than GBM [6], implying that PCNSL is associated with higher vascular permeability. This idea is consistent with previous studies conducted with DSC MRI and CT perfusion [8, 32]. Nevertheless, although advanced MRI techniques is necessitated for differentiating PCNSL from GBM when conventional MRI findings overlap in atypical cases, most previous studies have enrolled all PCNSL and GBM cases, regardless of their conventional MRI findings. We reasoned that confining our study to PCNSL and atypical GBM cases for which the conventional MRI findings overlapped would be better approach for determining if advanced MRI techniques are practical for discriminating PCNSL from GBM. Another issue to consider is reproducibility, which has been an issue in rCBV and K trans measurements. One recent study reported that the interobserver variability of rCBV measurement ranged from 24–67 %, depending on the normalization method and post-processing software used [10], and another study demonstrated that only one half of commercial programs showed good correlation between observed values and true values of perfusion MRI parameters [12]. Likewise, K trans requires pharmacokinetic model-based calculations that may vary depending on model selection, arterial input function, post-processing software, and selection among pre-bolus T1 measurements or a fixed T1 value [13–18]. In one recent study that explored the usefulness of DCE-MRI for determining recurrent high-grade glioma versus treatment effect [18], the mean K trans showed a wide interobserver variability of 49.8 % ± 286.1 % and poor interrater agreement for determining recurrent high-glioma versus treatment effect. However, in this previous study, short-delayed area under the time-to-signal intensity curve, a model-independent parameter of DCE-MRI, showed 14.7 % ± 28.2 % interobserver variability and moderate interrater agreement for determining recurrent high-glioma versus treatment. Thus, we chose the IAUC and ADC because these two parameters are operator-independent and exhibit high reproducibility. In addition, we minimized the user-dependent input that may have an effect on reproducibility such that the IAUC values were automatically calculated using an automatically determined wash-in phase. Thus, in our opinion, semiautomatic ROI segmentation of enhancing tumours was the only process that could have influenced the reproducibility of the IAUC values in this study.
The IAUC obtained from DCE-MRI is a model-independent parameter that is less likely to be affected by variations in sequence type, postprocessing pharmacokinetic model, and arterial input function [13, 19]. Previous studies showed that the IAUC correlated with the model-dependent parameters (i.e., vascular permeability, plasma volume, and extracellular-extraplasma volume) of DCE-MRI [14, 33, 34]. Although IAUC values are less specific for underlying physiology than the model-based parameters of DCE-MRI and rCBV, these results imply that the IAUC has the advantage of reflecting multiple characteristics of the tumour microenvironment, as well as high reproducibility. Thus, a number of studies have successfully used IAUC values for evaluating various diseases [13, 19, 23]. In regards to brain tumours, previous studies have demonstrated that the model-independent parameters of DCE-MRI, such as the IAUC at 30, 60, and 120 s and the ratio of the IAUC to the final area under the time-to-signal intensity curve, might be useful in the differentiation of radiation necrosis from recurrent GBM or high-grade glioma [13, 18, 19, 23, 24]. However, the diagnostic value of IAUC to discriminate PCNSL from GBM has not been previously established. In this study, we observed a higher IAUC for GBM than for PCNSL. Given that previous studies demonstrated that GBMs tend to show high perfusion (rCBV), whereas PCNSLs tend to show high permeability (K trans), the effect on IAUC values from different perfusion and permeability between GBM and PCNSL would have been opposite to each other. Our result implies that the IAUC difference is more likely to be attributed to the higher perfusion of GBM than the higher permeability of PCNSL. Although IAUC is a less specific parameter and the underlying histopathologic mechanisms driving the IAUC difference between PCNSL and GBM remain unclear, the IAUC values showed good diagnostic performance and added value to ADC. Moreover, IAUC values were highly reproducible, an aspect that is essential for practical implementation of advanced MRI. Our results indicate that the IAUC, in combination with ADC, is a practical and useful parameter for discriminating PCNSL from GBM when conventional MRI is insufficient. In our opinion, optimization and standardization can be achieved in this method.
Aside from the retrospective nature of the study and its small size, several major limitation should be mentioned. First, b = 600 s/mm2 instead of b = 1000 s/mm2 was used for ADC measurement. Thus, the perfusion effect may have been incorrectly measured, leading to overestimation of ADC. However, given the lower cellularity and higher perfusion of GBM, the effects of perfusion and cellularity on ADC values would be predicted to result in higher ADC values of GBM and lower ADC values of PCNSL. Thus, the perfusion effect resulting from low b values would not be expected to reduce the diagnostic performance of ADC to differentiate PCNSL. Subsequently, the added value of IAUC to ADC shown in our study might not be overestimated, even though the accuracy of the ADC values might have been reduced. Second, the IAUC and their cutoff values might be scanner- and method-dependent. Further study is mandatory to validate the reproducibility of IAUC on different scanners. However, once institutional standardization and optimization are achieved, IAUC can be a practical parameter, since IAUC in our study requires no user dependent input and yielded high reproducibility.
In conclusion, IAUC is a potentially useful imaging biomarker with added diagnostic value to ADC in the discrimination of PCNSL from atypical GBM, in which optimization and standardization can be achieved.
Abbreviations
- ADC:
-
apparent diffusion coefficient
- AUC:
-
area under the receiver operating characteristic curve
- DCE:
-
dynamic contrast-enhanced
- DSC:
-
dynamic susceptibility contrast-enhanced
- DTI:
-
diffusion tensor imaging
- GBM:
-
glioblastoma
- IAUC:
-
initial area under the curve
- PCNSL:
-
primary central nervous system lymphoma
- p10:
-
10th percentile
- p90:
-
90th percentile
- ROI:
-
region of interest
References
Schlegel U (2009) Primary CNS lymphoma. Ther Adv Neurol Disord 2:93–104
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Haldorsen IS, Espeland A, Larsson E-M (2011) Central nervous system lymphoma: characteristic findings on traditional and advanced imaging. AJNR Am J Neuroradiol 32:984–992
Al-Okaili RN, Krejza J, Woo JH et al (2007) Intraaxial Brain Masses: MR Imaging–based Diagnostic Strategy—Initial Experience. Radiology 243:539–550
Kickingereder P, Wiestler B, Sahm F et al (2014) Primary Central Nervous System Lymphoma and Atypical Glioblastoma: Multiparametric Differentiation by Using Diffusion-, Perfusion-, and Susceptibility-weighted MR Imaging. Radiology 272:843–850
Kickingereder P, Sahm F, Wiestler B et al (2014) Evaluation of Microvascular Permeability with Dynamic Contrast-Enhanced MRI for the Differentiation of Primary CNS Lymphoma and Glioblastoma: Radiologic-Pathologic Correlation. Am J Neuroradiol 35:1503–1508
Toh C-H, Castillo M, Wong A-C et al (2008) Primary cerebral lymphoma and glioblastoma multiforme: differences in diffusion characteristics evaluated with diffusion tensor imaging. Am J Neuroradiol 29:471–475
Toh CH, Wei K-C, Chang C-N et al (2013) Differentiation of Primary Central Nervous System Lymphomas and Glioblastomas: Comparisons of Diagnostic Performance of Dynamic Susceptibility Contrast-Enhanced Perfusion MR Imaging without and with Contrast-Leakage Correction. Am J Neuroradiol 34:1145–1149
Paulson ES, Schmainda KM (2008) Comparison of Dynamic Susceptibility-weighted Contrast-enhanced MR Methods: Recommendations for Measuring Relative Cerebral Blood Volume in Brain Tumors. Radiology 249:601–613
Prah MA, Stufflebeam SM, Paulson ES et al (2015) Repeatability of Standardized and Normalized Relative CBV in Patients with Newly Diagnosed Glioblastoma. Am J Neuroradiol 36:1654–1661
Essock-Burns E, Phillips JJ, Molinaro AM et al (2013) Comparison of DSC-MRI post-processing techniques in predicting microvascular histopathology in patients newly diagnosed with GBM. J Magn Reson Imaging 38:388–400
Kudo K, Christensen S, Sasaki M et al (2013) Accuracy and Reliability Assessment of CT and MR Perfusion Analysis Software Using a Digital Phantom. Radiology 267:201–211
Chung WJ, Kim HS, Kim N et al (2013) Recurrent Glioblastoma: Optimum Area under the Curve Method Derived from Dynamic Contrast-enhanced T1-weighted Perfusion MR Imaging. Radiology 269:561–568
Cheng H-LM (2009) Improved correlation to quantitative DCE-MRI pharmacokinetic parameters using a modified initial area under the uptake curve (mIAUC) approach. J Magn Reson Imaging 30:864–872
Beuzit L, Eliat P-A, Brun V et al (2015) Dynamic contrast-enhanced MRI: Study of inter-software accuracy and reproducibility using simulated and clinical data. J Magn Reson Imaging. doi:10.1002/jmri.25101
Tietze A, Mouridsen K, Mikkelsen IK (2015) The impact of reliable prebolus T 1 measurements or a fixed T 1 value in the assessment of glioma patients with dynamic contrast enhancing MRI. Neuroradiology 57:561–572
Fedorov A, Fluckiger J, Ayers GD et al (2014) A comparison of two methods for estimating DCE-MRI parameters via individual and cohort based AIFs in prostate cancer: A step towards practical implementation. Magn Reson Imaging 32:321–329
Hamilton JD, Lin J, Ison C et al (2015) Dynamic Contrast-Enhanced Perfusion Processing for Neuroradiologists: Model-Dependent Analysis May Not Be Necessary for Determining Recurrent High-Grade Glioma versus Treatment Effect. Am J Neuroradiol 36:686–693
Narang J, Jain R, Arbab AS et al (2011) Differentiating treatment-induced necrosis from recurrent/progressive brain tumor using nonmodel-based semiquantitative indices derived from dynamic contrast-enhanced T1-weighted MR perfusion. Neuro-Oncol 13:1037–1046
Akisik MF, Sandrasegaran K, Bu G et al (2010) Pancreatic Cancer: Utility of Dynamic Contrast-enhanced MR Imaging in Assessment of Antiangiogenic Therapy. Radiology 256:441–449
Zahra MA, Tan LT, Priest AN et al (2009) Semiquantitative and Quantitative Dynamic Contrast-Enhanced Magnetic Resonance Imaging Measurements Predict Radiation Response in Cervix Cancer. Int J Radiat Oncol 74:766–773
Rieber A, Brambs H-J, Gabelmann A et al (2002) Breast MRI for monitoring response of primary breast cancer to neo-adjuvant chemotherapy. Eur Radiol 12:1711–1719
Park JE, Kim HS, Goh MJ et al (2015) Pseudoprogression in Patients with Glioblastoma: Assessment by Using Volume-weighted Voxel-based Multiparametric Clustering of MR Imaging Data in an Independent Test Set. Radiology 275:792–802
Bisdas S, Naegele T, Ritz R et al (2011) Distinguishing recurrent high-grade gliomas from radiation injury: a pilot study using dynamic contrast-enhanced MR imaging. Acad Radiol 18:575–583
Ostrom QT, Gittleman H, Fulop J et al (2015) CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008-2012. Neuro-Oncol 17:iv1–iv62
Lee HY, Kim HS, Park JW et al (2013) Atypical Imaging Features of Epstein-Barr Virus–Positive Primary Central Nervous System Lymphomas in Patients without AIDS. Am J Neuroradiol 34:1562–1567
Kitai R, Matsuda K, Adachi E et al (2010) Epstein-Barr Virus-Associated Primary Central Nervous System Lymphoma in the Japanese Population. Neurol Med Chir (Tokyo) 50:114–118
Geddes JF, Bhattacharjee MB, Savage K et al (1992) Primary cerebral lymphoma: a study of 47 cases probed for Epstein-Barr virus genome. J Clin Pathol 45:587–590
Krogh-Jensen M, Johansen P, D’amore F (1998) Primary Central Nervous System Lymphomas in Immunocompetent Individuals: Histology, Epstein-Barr Virus Genome, Ki-67 Proliferation Index, p53 and bcl-2 Gene Expression. Leuk Lymphoma 30:131–142
Pencina MJ, D’ Agostino RB, D’ Agostino RB, Vasan RS (2008) Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat Med 27:157–172
Pencina MJ, D’Agostino RB, Steyerberg EW (2011) Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med 30:11–21
Warnke PC, Timmer J, Ostertag CB, Kopitzki K (2005) Capillary physiology and drug delivery in central nervous system lymphomas. Ann Neurol 57:136–139
Walker-Samuel S, Leach MO, Collins DJ (2006) Evaluation of response to treatment using DCE-MRI: the relationship between initial area under the gadolinium curve (IAUGC) and quantitative pharmacokinetic analysis. Phys Med Biol 51:3593
Evelhoch JL (1999) Key factors in the acquisition of contrast kinetic data for oncology. J Magn Reson Imaging 10:254–259
Acknowledgments
The scientific guarantor of this publication is Seung-Koo Lee. The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. No complex statistical methods were necessary for this paper. Institutional Review Board approval was obtained. Written informed consent was waived by the Institutional Review Board. Some study subjects or cohorts have been previously reported in the American Journal of Neuroradiology and Radiology as the following:
Choi YS, Kim DW, Lee S-K, et al (2015) The Added Prognostic Value of Preoperative Dynamic Contrast-Enhanced MRI Histogram Analysis in Patients with Glioblastoma: Analysis of Overall and Progression-Free Survival. AJNR Am J Neuroradiol 36:2235–2241.
Choi YS, Ahn SS, Kim DW, et al (2016) Incremental Prognostic Value of ADC Histogram Analysis over MGMT Promoter Methylation Status in Patients with Glioblastoma. Radiology doi: 10.1148/radiol.2016151913
Methodology: retrospective, observational, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, Y.S., Lee, HJ., Ahn, S.S. et al. Primary central nervous system lymphoma and atypical glioblastoma: differentiation using the initial area under the curve derived from dynamic contrast-enhanced MR and the apparent diffusion coefficient. Eur Radiol 27, 1344–1351 (2017). https://doi.org/10.1007/s00330-016-4484-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-016-4484-2