Abstract
Objectives
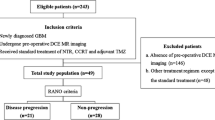
To prospectively explore the value of dynamic contrast-enhanced magnetic resonance imaging (DCE–MRI) in predicting the progression of enhancing lesions persisting after standard treatment in patients with surgically resected glioblastoma (GBM).
Methods
Forty-seven GBM patients, who underwent near-total tumorectomy followed by concurrent chemoradiation therapy (CCRT) with temozolomide (TMZ) between May 2014 and February 2016, were enrolled. Twenty-four patients were finally analyzed for measurable enhancing lesions persisting after standard treatment. DCE-MRI parameters were calculated at enhancing lesions. Mann–Whitney U tests and multivariable stepwise logistic regression were used to compare parameters between progression (n = 16) and non-progression (n = 8) groups.
Results
Mean Ktrans and ve were significantly lower in progression than in non-progression (P = 0.037 and P = 0.037, respectively). The 5th percentile of the cumulative Ktrans histogram was also significantly lower in the progression than in non-progression group (P = 0.017). Mean ve was the only independent predictor of progression (P = 0.007), with a sensitivity of 100%, specificity of 63%, and an overall accuracy of 88% at a cut-off value of 0.873.
Conclusions
DCE-MRI may help predict the progression of enhancing lesions persisting after the completion of standard treatment in patients with surgically resected GBM, with mean ve serving as an independent predictor of progression.
Key points
• Enhancing lesions may persist after standard treatment in GBM patients.
• DCE-MRI may help predict the progression of the enhancing lesions.
• Mean K trans and v e were lower in progression than in non-progression group.
• DCE-MRI may help identify patients requiring close follow-up after standard treatment.
• DCE-MRI may help plan treatment strategies for GBM patients.




Similar content being viewed by others
Abbreviations
- AIF:
-
Arterial input function
- BBB:
-
Blood–brain barrier
- CBV:
-
Cerebral blood volume
- CCRT:
-
Concurrent radiation therapy and chemotherapy
- CI:
-
Confidence interval
- DCE:
-
Dynamic contrast-enhanced
- F-FMISO:
-
18F-fluoromisonidazole
- FLAIR:
-
Fluid-attenuated inversion recovery sequence
- FOV:
-
Field of view
- GBM:
-
Glioblastoma
- IQR:
-
Interquartile range
- MGMT:
-
O6-Methylguanine DNA methyltransferase
- MPRAGE:
-
Magnetization-prepared rapid acquisition gradient echo
- NEX:
-
Number of excitations
- RANO:
-
Response assessment in neuro-oncology
- ROC:
-
Receiver operating characteristic
- ROI:
-
Region of interest
- TE:
-
Echo time
- TI:
-
Inversion time
- TMZ:
-
Temozolomide
- TR:
-
Repetition time
- T1WI:
-
T1-weighted imaging
- T2WI:
-
T2-weighted imaging
References
Haroon HA, Buckley DL, Patankar TA et al (2004) A comparison of Ktrans measurements obtained with conventional and first pass pharmacokinetic models in human gliomas. J Magn Reson Imaging 19:527–536
Harrer JU, Parker GJ, Haroon HA et al (2004) Comparative study of methods for determining vascular permeability and blood volume in human gliomas. J Magn Reson Imaging 20:748–757
Tofts PS, Brix G, Buckley DL et al (1999) Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging 10:223–232
Jia Z, Geng D, Xie T, Zhang J, Liu Y (2012) Quantitative analysis of neovascular permeability in glioma by dynamic contrast-enhanced MR imaging. J Clin Neurosci 19:820–823
Jung SC, Yeom JA, Kim JH et al (2014) Glioma: Application of histogram analysis of pharmacokinetic parameters from T1-weighted dynamic contrast-enhanced MR imaging to tumor grading. AJNR Am J Neuroradiol 35:1103–1110
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Yun TJ, Park CK, Kim TM et al (2015) Glioblastoma treated with concurrent radiation therapy and temozolomide chemotherapy: differentiation of true progression from pseudoprogression with quantitative dynamic contrast-enhanced MR imaging. Radiology 274:830–840
Kim JH, Choi SH, Ryoo I et al (2014) Prognosis prediction of measurable enhancing lesion after completion of standard concomitant chemoradiotherapy and adjuvant temozolomide in glioblastoma patients: application of dynamic susceptibility contrast perfusion and diffusion-weighted imaging. PLoS One 9, e113587
Wen PY, Macdonald DR, Reardon DA et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972
Tofts PS, Kermode AG (1991) Measurement of the blood-brain barrier permeability and leakage space using dynamic MR imaging. 1. Fundamental concepts. Magn Reson Med 17:357–367
Haacke EM, Filleti CL, Gattu R et al (2007) New algorithm for quantifying vascular changes in dynamic contrast-enhanced MRI independent of absolute T1 values. Magn Reson Med 58:463–472
Pluim JP, Maintz JB, Viergever MA (2003) Mutual-information-based registration of medical images: a survey. IEEE Trans Med Imaging 22:986–1004
Sundar H, Shen D, Biros G, Xu C, Davatzikos C (2007) Robust computation of mutual information using spatially adaptive meshes. Med Image Comput Comput Assist Interv 10:950–958
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Hauck WW, Miike R (1991) A proposal for examining and reporting stepwise regressions. Stat Med 10:711–715
Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ (2010) Structure and function of the blood-brain barrier. Neurobiol Dis 37:13–25
de Vries NA, Beijnen JH, Boogerd W, van Tellingen O (2006) Blood-brain barrier and chemotherapeutic treatment of brain tumors. Expert Rev Neurother 6:1199–1209
Dewhirst MW (1998) Concepts of oxygen transport at the microcirculatory level. Semin Radiat Oncol 8:143–150
Gulledge CJ, Dewhirst MW (1996) Tumor oxygenation: a matter of supply and demand. Anticancer Res 16:741–749
Perini R, Choe R, Yodh AG, Sehgal C, Divgi CR, Rosen MA (2008) Non-invasive assessment of tumor neovasculature: techniques and clinical applications. Cancer Metastasis Rev 27:615–630
Pettersen EO, Ebbesen P, Gieling RG et al (2015) Targeting tumour hypoxia to prevent cancer metastasis. From biology, biosensing and technology to drug development: the METOXIA consortium. J Enzyme Inhib Med Chem 30:689–721
Barsoum IB, Smallwood CA, Siemens DR, Graham CH (2014) A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res 74:665–674
Calcinotto A, Filipazzi P, Grioni M et al (2012) Modulation of microenvironment acidity reverses anergy in human and murine tumor-infiltrating T lymphocytes. Cancer Res 72:2746–2756
Casazza A, Di Conza G, Wenes M, Finisguerra V, Deschoemaeker S, Mazzone M (2014) Tumor stroma: a complexity dictated by the hypoxic tumor microenvironment. Oncogene 33:1743–1754
Colegio OR, Chu NQ, Szabo AL et al (2014) Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 513:559–563
Finger EC, Giaccia AJ (2010) Hypoxia, inflammation, and the tumor microenvironment in metastatic disease. Cancer Metastasis Rev 29:285–293
Gabrilovich DI, Ostrand-Rosenberg S, Bronte V (2012) Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 12:253–268
Hanahan D, Coussens LM (2012) Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21:309–322
Keith B, Johnson RS, Simon MC (2012) HIF1alpha and HIF2alpha: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer 12:9–22
Motz GT, Coukos G (2013) Deciphering and reversing tumor immune suppression. Immunity 39:61–73
Noy R, Pollard JW (2014) Tumor-associated macrophages: from mechanisms to therapy. Immunity 41:49–61
Palazon A, Aragones J, Morales-Kastresana A, de Landazuri MO, Melero I (2012) Molecular pathways: hypoxia response in immune cells fighting or promoting cancer. Clin Cancer Res 18:1207–1213
Semenza GL (2014) Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu Rev Pathol 9:47–71
Wilson WR, Hay MP (2011) Targeting hypoxia in cancer therapy. Nat Rev Cancer 11:393–410
Kikuchi M, Yamane T, Shinohara S et al (2011) 18F-fluoromisonidazole positron emission tomography before treatment is a predictor of radiotherapy outcome and survival prognosis in patients with head and neck squamous cell carcinoma. Ann Nucl Med 25:625–633
Kobayashi H, Hirata K, Yamaguchi S, Terasaka S, Shiga T, Houkin K (2013) Usefulness of FMISO-PET for glioma analysis. Neurol Med Chir (Tokyo) 53:773–778
Krohn KA, Link JM, Mason RP (2008) Molecular imaging of hypoxia. J Nucl Med 49(Suppl 2):129S–148S
Rischin D, Hicks RJ, Fisher R et al (2006) Prognostic significance of [18F]-misonidazole positron emission tomography-detected tumor hypoxia in patients with advanced head and neck cancer randomly assigned to chemoradiation with or without tirapazamine: a substudy of Trans-Tasman Radiation Oncology Group Study 98.02. J Clin Oncol 24:2098–2104
Cooper RA, Carrington BM, Loncaster JA et al (2000) Tumour oxygenation levels correlate with dynamic contrast-enhanced magnetic resonance imaging parameters in carcinoma of the cervix. Radiother Oncol 57:53–59
Egeland TA, Gulliksrud K, Gaustad JV, Mathiesen B, Rofstad EK (2012) Dynamic contrast-enhanced-MRI of tumor hypoxia. Magn Reson Med 67:519–530
Jensen RL, Mumert ML, Gillespie DL, Kinney AY, Schabel MC, Salzman KL (2014) Preoperative dynamic contrast-enhanced MRI correlates with molecular markers of hypoxia and vascularity in specific areas of intratumoral microenvironment and is predictive of patient outcome. Neuro Oncol 16:280–291
Rajendran JG, Krohn KA (2015) F-18 fluoromisonidazole for imaging tumor hypoxia: imaging the microenvironment for personalized cancer therapy. Semin Nucl Med 45:151–162
Cao VT, Jung TY, Jung S et al (2009) He correlation and prognostic significance of MGMT promoter methylation and MGMT protein in glioblastomas. Neurosurgery 65:866–875
Acknowledgements
The scientific guarantor of this publication is Seung Hong Choi. The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. This study was supported by a grant from Bayer Healthcare, the Korea Healthcare technology R&D Projects, Ministry for Health, Welfare & Family Affairs (HI16C1111), by the Brain Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2016M3C7A1914002), by Creative-Pioneering Researchers Program through Seoul National University (SNU), and by project code (IBS-R006-D1).
One of the authors (R.E.Y.) has significant statistical expertise. Institutional review board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study.
Some study subjects or cohorts (n = 10) have been previously reported in “Glioblastoma treated with concurrent radiation therapy and temozolomide chemotherapy: differentiation of true progression from pseudoprogression with quantitative dynamic contrast-enhanced MR imaging” (Radiology 2015;274(3):830–40). However, the main focus of the present study was to evaluate DCE-MRI findings of the patients at a different time point (i.e., after the completion of standard treatment) and to explore whether DCE pharmacokinetic parameters may predict the progression of enhancing lesions persisting after the completion of standard treatment in the patients with surgically resected GBM.
Methodology: prospective, case-control study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoo, RE., Choi, S.H., Kim, T.M. et al. Dynamic contrast-enhanced MR imaging in predicting progression of enhancing lesions persisting after standard treatment in glioblastoma patients: a prospective study. Eur Radiol 27, 3156–3166 (2017). https://doi.org/10.1007/s00330-016-4692-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-016-4692-9