Abstract
Objective
We aimed to identify optimal machine-learning methods for preoperative differentiation of sacral chordoma (SC) and sacral giant cell tumour (SGCT) based on 3D non-enhanced computed tomography (CT) and CT-enhanced (CTE) features.
Methods
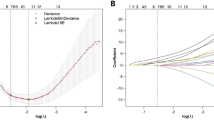
A total of 95 patients were divided into a training set and a validation set. Three best feature selection methods (Relief, least absolute shrinkage and selection operator (LASSO) and Random Forest (RF)) and three classification methods, including generalised linear models (GLM), support vector machines (SVM) and RF, were compared for their performance in distinguishing SC and SGCT. The performance of the radiomics model was investigated via area under the receiver-operating characteristic curve (AUC) and accuracy (ACC) analysis.
Results
The selection method LASSO + classifier GLM had the highest AUC of 0.984 and ACC of 0.897 in the validating set, followed by Relief + GLM (AUC = 0.909, ACC = 0.862) and LASSO + SVM (AUC = 0.900, ACC = 0.862) based on CTE features. For CT features, RF + GLM had the highest AUC of 0.889, while LASSO + GLM achieved a high ACC of 0.793 in the validating set. Regardless of the methods, CTE features significantly outperformed those from CT for the differentiation of SC and SGCT (ZAUC = -3.029, ZACC = -4.553; p < 0.05).
Conclusions
Our study demonstrated CTE features performed better than CT features. The selection method LASSO + classifier GLM had the best performance in differentiation of SC and SGCT, which could enhance the application of radiomics methods in sacral tumours.
Key Points
• Sacral chordoma and sacral giant cell tumour are the two most common primary tumours of the sacrum with many common clinical and imaging characteristics.
• A radiomics model helps clinicians to identify the histology of a sacral tumour.
• CTE features should be preferred.



Similar content being viewed by others
Abbreviations
- ACC:
-
Accuracy
- AUC:
-
Area under the receiver-operating characteristic curve
- CT:
-
Computed tomography
- CTE:
-
Computed tomography enhanced
- FOV:
-
Field of view
- GLM:
-
Generalised linear models
- ICC:
-
Intra- and interclass correlation coefficients
- LASSO:
-
Least absolute shrinkage and selection operator
- MDCT:
-
Multi-detector row CT
- PACS:
-
Picture archiving and communication system
- RF:
-
Random Forest
- ROIs:
-
Regions of interest
- SC:
-
Sacral chordoma
- SGCT:
-
Sacral giant cell tumour
- SVM:
-
Support vector machines
References
Thornton E, Krajewski KM, O'Regan KN, Giardino AA, Jagannathan JP, Ramaiya N (2012) Imaging features of primary and secondary malignant tumours of the sacrum. Br J Radiol 85(1011):279–286
Gerber S, Ollivier L, Leclère J et al (2008) Imaging of sacral tumours. Skeletal Radiol 37(4):277–289
Ji T, Guo W, Yang R, Tang X, Wang Y, Huang L (2017) What are the conditional survival and functional outcomes after surgical treatment of 115 patients with sacral chordoma? Clin Orthop Relat Res 475(3):620–630
Si MJ, Wang CS, Ding XY et al (2013) Differentiation of primary chordoma, giant cell tumor and schwannoma of the sacrum by CT and MRI. Eur J Radiol 82(12):2309–2315
Jamshidi K, Bagherifard A, Mirzaei A, Bahrabadi M (2017) Giant cell tumor of the sacrum: series of 19 patients and review of the literature. Arch Bone Jt Surg 5(6):443–450
Avanzo M, Stancanello J, El Naqa I (2017) Beyond imaging: the promise of radiomics. Phys Med 38:122–139
Ruosi C, Colella G, Di Donato SL, Granata F, Di Salvatore MG, Fazioli F (2015) Surgical treatment of sacral chordoma: survival and prognostic factors. Eur Spine J 24(Suppl 7):912–917
Vallières M, Freeman CR, Skamene SR, El Naqa I (2015) A radiomics model from joint FDG-PET and MRI texture features for the prediction of lung metastases in soft-tissue sarcomas of the extremities. Phys Med Biol 60:5471–5496
Shen C, Liu Z, Guan M et al (2017) 2D and 3D CT radiomics features prognostic performance comparison in non-small cell lung cancer. Transl Oncol 10(6):886–894
Bowen SR, Yuh WTC, Hippe DS et al (2018) Tumor radiomic heterogeneity: multiparametric functional imaging to characterize variability and predict response following cervical cancer radiation therapy. J Magn Reson Imaging 47(5):1388–1396
Larue RT, Defraene G, De Ruysscher D, Lambin P, van Elmpt W (2017) Quantitative radiomics studies for tissue characterization: a review of technology and methodological procedures. Br J Radiol 90(1070):20160665
Yip SS, Aerts HJ (2016) Applications and limitations of radiomics. Phys Med Biol 61(13):R150–R166
Lambin P, Leijenaar RTH, Deist TM et al (2017) Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 14(12):749–762
Kickingereder P, Burth S, Wick A et al (2016) Radiomic profiling of glioblastoma: identifying an imaging predictor of patient survival with improved performance over established clinical and radiologic risk models. Radiology 280(3):880–889
Liu J, Mao Y, Li Z et al (2016) Use of texture analysis based on contrast-enhanced MRI to predict treatment response to chemoradiotherapy in nasopharyngeal carcinoma. J Magn Reson Imaging 44:445–455
Lubner MG, Smith AD, Sandrasegaran K, Sahani DV, Pickhardt PJ (2017) CT texture analysis: definitions, applications, biologic correlates, and challenges. Radiographics 37(5):1483–1503
Cha KH, Hadjiiski L, Chan HP et al (2017) Bladder cancer treatment response assessment in CT using radiomics with deep-learning. Sci Rep 7(1):8738
Pota M, Scalco E, Sanguineti G et al (2017) Early prediction of radiotherapy-induced parotid shrinkage and toxicity based on CT radiomics and fuzzy classification. Artif Intell Med 81:41–53
Bashir U, Azad G, Siddique MM et al (2017) The effects of segmentation algorithms on the measurement of 18F-FDG PET texture parameters in non-small cell lung cancer. EJNMMI Res 7(1):60
Tixier F, Groves AM, Goh V et al (2014) Correlation of intra-tumor (18)F-FDG uptake heterogeneity indices with perfusion CT derived parameters in colorectal cancer. PLoS One 9:e99567
Wu W, Parmar C, Grossmann P et al (2016) Exploratory study to identify radiomics classifiers for lung cancer histology. Front Oncol 6:71
Parmar C, Grossmann P, Bussink J, Lambin P, Aerts HJ (2015) Machine learning methods for quantitative radiomic biomarkers. Sci Rep 5:13087
Zhang B, He X, Ouyang F et al (2017) Radiomic machine-learning classifiers for prognostic biomarkers of advanced nasopharyngeal carcinoma. Cancer Lett 403:21–27
Wu S, Zheng J, Li Y et al (2017) A radiomics nomogram for the preoperative prediction of lymph node metastasis in bladder cancer. Clin Cancer Res 23(22):6904–6911
Zhu X, Dong D, Chen Z et al (2018) Radiomic signature as a diagnostic factor for histologic subtype classification of non-small cell lung cancer. Eur Radiol. https://doi.org/10.1007/s00330-017-5221-1
Sun Y, Hu P, Wang J et al (2018) Radiomic features of pretreatment MRI could identify T stage in patients with rectal cancer: Preliminary findings. J Magn Reson Imaging. https://doi.org/10.1002/jmri.25969
Hawkins SH, Korecki JN, Balagurunathan Y et al (2014) Predicting outcomes of nonsmall cell lung cancer using CT image features. IEEE Access 2:1418–1426
Parmar C, Grossmann P, Rietveld D, Rietbergen MM, Lambin P, Aerts HJ (2015) Radiomic machine-learning classifiers for prognostic biomarkers of head and neck cancer. Front Oncol 5:272
Zhang B, Tian J, Dong D et al (2017) Radiomics features of multiparametric MRI as novel prognostic factors in advanced nasopharyngeal carcinoma. Clin Cancer Res 23(15):4259–4269
Yuan M, Zhang YD, Pu XH et al (2017) Comparison of a radiomic biomarker with volumetric analysis for decoding tumour phenotypes of lung adenocarcinoma with different disease-specific survival. Eur Radiol 27(11):4857–4865
Asl BM, Setarehdan SK, Mohebbi M (2008) Support vector machine-based arrhythmia classification using reduced features of heart rate variability signal. Artif Intell Med 44(1):51–64
Parekh V, Jacobs MA (2016) Radiomics: a new application from established techniques. Expert Rev Precis Med Drug Dev 1(2):207–226
Breiman L (2001) Random forests. Mach Learn 45(1):5–32
Fernández-Delgado M, Cernadas E, Barro S, Amorim D (2014) Do we need hundreds of classifiers to solve real world classification problems? J Mach Learn Res:3133-3181
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Jiangfen Wu.
Conflict of interest
The authors of this article declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Retrospective
• Diagnostic or prognostic study
• Performed at one institution
Rights and permissions
About this article
Cite this article
Yin, P., Mao, N., Zhao, C. et al. Comparison of radiomics machine-learning classifiers and feature selection for differentiation of sacral chordoma and sacral giant cell tumour based on 3D computed tomography features. Eur Radiol 29, 1841–1847 (2019). https://doi.org/10.1007/s00330-018-5730-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5730-6