Abstract
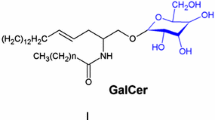
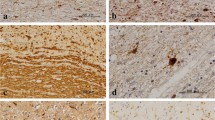
Inherited deficiency for the lysosomal enzyme arylsulfatase A (ASA) leads to lysosomal storage of sulfatides and to dramatic demyelination in the CNS of humans (metachromatic leukodystrophy, MLD). As an animal model, ASA–/– mice have previously been generated by disruption of the ASA gene and are known to develop lysosomal sulfatide storage similar to that in human MLD, and, moreover, to become deaf because of degeneration of the primary neurons of the auditory pathway. The present study deals with the cellular and topographic distribution of sulfatide storage throughout the CNS of ASA–/– mice between a few days and 24 months of age. Sulfatide accumulation was detected on the ultrastructural level and by histochemical staining with alcian blue. Sulfatide storage was found in oligodendroglia and neurons in young mice, and in activated microglia (phagocytes) in adult mice. Neuronal sulfatide storage was most prominent in many nuclei of the medulla oblongata and pons, and in several nuclei of midbrain and forebrain. Sulfatide-storing phagocytes were most frequent in the white matter tracts of aged ASA–/– mice, whereas no widespread demyelination was obvious. Loss of neurons was found in two nuclei of the auditory pathway of aged ASA–/– mice (ventral cochlear nucleus and nucleus of trapezoid body). The distributional pattern of sulfatide storage throughout the CNS of ASA–/– mice largely corresponds to data reported for human MLD. An important difference, however, which remains unexplained at present, is the absence of obvious demyelination from the CNS of ASA–/– mice up to the age of 2 years.








Similar content being viewed by others
References
Alves D, Pires MM, Guimaraes A, Miranda MC (1986) Four cases of the late onset metachromatic leukodystrophy in a family: clinical, biochemical and neuropathological studies. J Neuropathol Neurosurg Psychiatry 49:1417–1422
Bischoff A, Ulrich J (1967) Amaurotische Idiotie in Verbindung mit metachromatischer Leukodystrophie: Übergangsform oder Kombination? Acta Neuropathol (Berl) 8:292–308
Castleman B, Kibbee BU (1962) Case records of the Massachusetts General Hospital. N Engl J Med 267:1198–1204
Coenen R, Gieselmann V, Lüllmann-Rauch R (2001) Morphological alterations in the inner ear of the arylsulfatase A-deficient mouse. Acta Neuropathol 101:491–498
Consiglio A, Quattrini A, Martino S, Bensadoun JC, Dolcetta D, Trojani A, Benaglia G, Marchesini S, Cestari V, Oliviero A, Bordignion C, Naldini L (2001) In vivo therapy of metachromatic leukodystrophy by lentiviral vectors: correction of neuropathology and protection against learning impairments in affected mice. Nat Med 7:310–316
D’Hooge R, Coenen R, Gieselmann V, Lüllmann-Rauch R, DeDeyn PP (1999). Decline in brain stem auditory-evoked potentials coincides with loss of spiral ganglion cells in arylsulfatase A-deficient mice. Brain Res 847:352–356
D’Hooge R, Hartmann D, Manil J, Colin F, Gieselmann V, DeDeyn PP (1999) Neuromotor alterations and cerebellar deficits in aged arylsulfatase A-deficient transgenic mice. Neurosci Lett 273:93–96
D’Hooge R, Van Dam D, Frank F, Gieselmann V, DeDeyn PP (2001) Hyperactivity, neuromotor defects and impaired learning and memory in a mouse model for metachromatic leukodystrophy. Brain Res 907:35–43
Figura K von, Gieselmann V, Jaeken J (2001) Metachromatic leukodystrophy. In: Scriver CR, Beaudet, AL, Valle D, Sly WS (eds) The metabolic and molecular bases of inherited disease, 8th edn. McGraw-Hill, New York, pp 3695–3724
Grégoire A, Perier O, Dustin P (1966). Metachromatic leukodystrophy, an electron microscopic study. J Neuropathol Exp Neurol 25:617–636
Hagberg B, Sourander P, Svennerholm L (1962) Sulfatide lipidosis in childhood. Am J Dis Child 104:644–656
Harrison JM, Feldman ML (1970) Anatomical aspects of the cochlear nucleus and superior olive complex. In: Neff WD (ed) Contributions to sensory physiology, vol 4. Acad Press, New York, pp 95–142
Hashisaki GT, Rubel EW (1989) Effects of unilateral cochlear removal on anteroventral cochlear nucleus neurons in developing gerbils. J Comp Neurol 283:465–473
Hess B, Saftig P, Hartmann D, Coenen R, Lüllmann-Rauch R, Goebel HH, Evers M, Figura K von, D’Hooge R, Nagels G, De Deyn P, Peters C, Gieselmann V (1996) Phenotype of arylsulfatase A-deficient mice: relationship to human metachromatic leukodystrophy. Proc Natl Acad Sci USA 93:14821–14826
Lüllmann-Rauch R, Matzner U, Franken S, Hartmann D, Gieselmann V (2001) Lysosomal sulfolipid storage in the kidneys of mice deficient for arylsulfatase A (ASA) and double-knockout mice deficient for ASA and galactosylceramide synthase. Histochem Cell Biol 116:161–169
Martin JJ, Ceuterick C, Mercelis R, Joris C (1982) Pathology of the peripheral nerves in metachromatic leukodytrophy. J Neurol Sci 53:95–112
Meier C, Bischoff A (1976) Sequence of morphological alterations in nervous system of metachromatic leukodytrophy. Acta Neuropathol (Berl) 36:369–379
Moore DR (1990) Auditory brainstem of the ferret: early cessation of developmental sensitivity of neurons in the cochlear nucleus to removal of the cochlea. J Comp Neurol 302:810–823
Norton WT, Poduslo SE (1982) Biochemical studies of metachromatic leukodystrophy in three siblings. Acta Neuropathol (Berl) 57:188–196
Osen KK (1969) The cytoarchitecture of the cochlear nuclei in the cat. J Comp Neurol 136:453–484
Paxinos G, Franklin KBJ (2001) The mouse brain in stereotaxic coordinates, 2nd edn, Academic Press, New York
Peiffer J (1959) Über die metachromatischen Leukodystrophien (Typ Scholz). Arch Psychiatr Z Neurol 199:386–416
Peng L, Suzuki K (1987) Ultrastructural study of neurons in metachromatic leukodystrophy. Clin Neuropathol 6:224–230
Peters A, Palay SL, Webster HF (1976) The fine structure of the nervous system. Saunders, Philadelphia
Poduslo SE, Tennekoon G, Price D, Miller K, McKhann GM (1976) Fetal metachromatic leukodystrophy: pathology, biochemistry and a study of in vitro enzyme replacement in CNS tissue. J Neuropathol Exp Neurol 35:622–632
Résibois-Grégoire A (1967) Electron microscopic studies of metachromatic leukodystrophy. II. Compound nature of the inclusions. Acta Neuropathol (Berl) 9:244–253
Schott I, Hartmann D, Gieselmann V, Lüllmann-Rauch R (2001) Sulfatide storage in visceral organs of arylsulfatase A-deficient mice. Virchows Arch 439:90–96
Scott JE (1970) Histochemistry of alcian blue. I. Metachromasia of alcian blue, astrablau and other cationic phthalocyanin dyes. Histochemistry 21:277–285
Scott JE, Dorling J (1965) Differential staining of acid glycosaminoglycans (mucopolysaccharides) with alcian blue in salt solutions. Histochemie 5:221–233
Scott JE, Orford CR, Hughes EW (1981) Proteoglycan-collagen arrangements in developing rat tail tendon. Biochem J 195:573–581
Trune DR (1982) Influence of neonatal cochlear removal on the development of mouse cochlear nucleus: number, size and density of its neurons. J Comp Neurol 209:409–424
Yamano T, Ohta S, Shimada M, Okada S, Yotaka T, Sugita T, Yabuuchi H (1980) Neuronal depletion of cerebellum in late infantile metachromatic leukodystrophy. Brain Dev 2:359–369
Acknowledgements
The excellent technical assistance of Dagmar Niemeier is gratefully acknowledged. We thank Clemens Franke for assistance with the graphical work and Heide Siebke and Heidi Waluk for the photographical work. This study was financially supported by the Deutsche Forschungsgemeinschaft (Grant no. Lu 172-8/2)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wittke, D., Hartmann, D., Gieselmann, V. et al. Lysosomal sulfatide storage in the brain of arylsulfatase A-deficient mice: cellular alterations and topographic distribution. Acta Neuropathol 108, 261–271 (2004). https://doi.org/10.1007/s00401-004-0883-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-004-0883-6