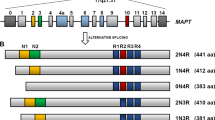
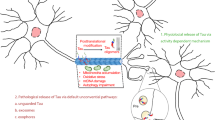
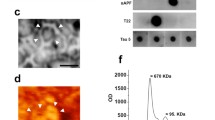
Abstract
Alzheimer disease (AD) and related tauopathies are histopathologically characterized by a specific type of slow and progressive neurodegeneration, which involves the abnormal hyperphosphorylation of the microtubule associated protein (MAP) tau. This hallmark, called neurofibrillary degeneration, is seen as neurofibrillary tangles, neuropil threads, and dystrophic neurites and is apparently required for the clinical expression of AD, and in related tauopathies it leads to dementia in the absence of amyloid plaques. While normal tau promotes assembly and stabilizes microtubules, the non-fibrillized, abnormally hyperphosphorylated tau sequesters normal tau, MAP1 and MAP2, and disrupts microtubules. The abnormal hyperphosphorylation of tau, which can be generated by catalysis of several different combinations of protein kinases, also promotes its misfolding, decrease in turnover, and self-assembly into tangles of paired helical and or straight filaments. Some of the abnormally hyperphosphorylated tau ends up both amino and C-terminally truncated. Disruption of microtubules by the non-fibrillized abnormally hyperphosphorylated tau as well as its aggregation as neurofibrillary tangles probably impair axoplasmic flow and lead to slow progressive retrograde degeneration and loss of connectivity of the affected neurons. Among the phosphatases, which regulate the phosphorylation of tau, protein phosphatase-2A (PP2A), the activity of which is down-regulated in AD brain, is by far the major enzyme. The two inhibitors of PP-2A, I PP2A1 and I PP2A2 , which are overexpressed in AD, might be responsible for the decreased phosphatase activity. AD is multifactorial and heterogeneous and involves more than one etiopathogenic mechanism.



Similar content being viewed by others
Abbreviations
- AD:
-
Alzheimer disease
- PHF:
-
Paired helical filaments
- SF:
-
Straight filaments
References
Alafuzoff I, Iqbal K, Friden H, Adolfsson R, Winblad B (1987) Histopathological criteria for progressive dementia disorders: clinical-pathological correlation and classification by multivariate data analysis. Acta Neuropathol 74:209–225. doi:10.1007/BF00688184
Alonso AD, Grundke-Iqbal I, Iqbal K (1996) Alzheimer’s disease hyperphosphorylated tau sequesters normal tau into tangles of filaments and disassembles microtubules. Nat Med 2:783–787. doi:10.1038/nm0796-783
Alonso AD, Zaidi T, Grundke-Iqbal I, Iqbal K (1994) Role of abnormally phosphorylated tau in the breakdown of microtubules in Alzheimer disease. Proc Natl Acad Sci USA 91:5562–5566. doi:10.1073/pnas.91.12.5562
Alonso AD, Grundke-Iqbal I, Barra HS, Iqbal K (1997) Abnormal phosphorylation of tau and the mechanism of Alzheimer neurofibrillary degeneration: sequestration of microtubule-associated proteins 1 and 2 and the disassembly of microtubules by the abnormal tau. Proc Natl Acad Sci USA 94:298–303. doi:10.1073/pnas.94.1.298
Alonso AD, Li B, Grundke-Iqbal I, Iqbal K (2006) Polymerization of hyperphosphorylated tau into filaments eliminates its inhibitory activity. Proc Natl Acad Sci USA 23:8864–8869. doi:10.1073/pnas.0603214103
Alonso AD, Zaidi T, Novak M, Grundke-Iqbal I, Iqbal K (2001) Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc Natl Acad Sci USA 98:6923–6928. doi:10.1073/pnas.121119298
Alonso AD, Mederlyova A, Novak M, Grundke-Iqbal I, Iqbal K (2004) Promotion of hyperphosphorylation by frontotemporal dementia tau mutations. J Biol Chem 279:34873–34881. doi:10.1074/jbc.M405131200
Alonso AD, Zaidi T, Novak M et al (2001) Interaction of tau isoforms with Alzheimer’s disease abnormally hyperphosphorylated tau and in vitro phosphorylation into the disease-like protein. J Biol Chem 276:37967–37973. doi:10.1074/jbc.M006497200
An WL, Cowburn RF, Li L et al (2003) Up-regulation of phosphorylated/activated p70 S6 kinase and its relationship to neurofibrillary pathology in Alzheimer’s disease. Am J Pathol 163:591–607
Anderton BH, Betts J, Blackstock WP et al (2001) Sites of phosphorylation in tau and factors affecting their regulation. Biochem Soc Symp 67:73–80
Andorfer C, Acker CM, Kress Y et al (2005) Cell-cycle reentry and cell death in transgenic mice expressing nonmutant human tau isoforms. J Neurosci 25:5446–5454. doi:10.1523/JNEUROSCI.4637-04.2005
Arendt T, Stieler J, Strijkstra AM et al (2003) Reversible paired helical filament-like phosphorylation of tau is an adaptive process associated with neuronal plasticity in hibernating animals. J Neurosci 23:6972–6981
Arnold CS, Johnson GV, Cole RN et al (1996) The microtubule-associated protein tau is extensively modified with O-linked N-acetylglucosamine. J Biol Chem 271:28741–28744. doi:10.1074/jbc.271.46.28741
Arrasate M, Perez M, Armas-Portela R, Avila J (1999) Polymerization of tau peptides into fibrillar structures The effect of FTDP-17 mutations. FEBS Lett 446:199–202. doi:10.1016/S0014-5793(99)00210-0
Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT (1992) Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology 42:631–639
Baki L, Shioi J, Wen P et al (2004) PS1 activates PI3K thus inhibiting GSK-3 activity and tau overphosphorylation: effects of FAD mutations. EMBO J 23:2586–2596. doi:10.1038/sj.emboj.7600251
Bancher C, Grundke-Iqbal I, Iqbal K et al (1991) Abnormal phosphorylation of tau precedes ubiquitination in neurofibrillary pathology of Alzheimer disease. Brain Res 539:11–18. doi:10.1016/0006-8993(91)90681-K
Bancher C, Brunner C, Lassmann H et al (1989) Accumulation of abnormally phosphorylated tau precedes the formation of neurofibrillary tangles in Alzheimer’s disease. Brain Res 477:90–99. doi:10.1016/0006-8993(89)91396-6
Bennecib M, Gong CX, Grundke-Iqbal I, Iqbal K (2001) Inhibition of PP-2A upregulates CaMKII in rat forebrain and induces hyperphosphorylation of tau at Ser 262/356. FEBS Lett 490:15–22. doi:10.1016/S0014-5793(01)02127-5
Bhaskar K, Yen SH, Lee G (2005) Disease-related modifications in tau affect the interaction between Fyn and Tau. J Biol Chem 280:35119–35125. doi:10.1074/jbc.M505895200
Cash AD, Aliev G, Siedlak SL et al (2003) Microtubule reduction in Alzheimer’s disease and aging is independent of tau filament formation. Am J Pathol 162:1623–1627
Chen S, Grundke-Iqbal I, Iqbal K (2006) I PP2A1 and I PP2A2 affect tau phosphorylation via association with the catalytic subunit of protein phosphatase 2A. Alzheimers Dement 2:S471. doi:10.1016/j.jalz.2006.05.1599
Chen S, Li B, Grundke-Iqbal I, Iqbal K (2008) I1PP2A affects tau phosphorylation via association with the catalytic subunit of protein phosphatase 2A. J Biol Chem 283:10513–10521. doi:10.1074/jbc.M709852200
Cheng LY, Wang JZ, Gong CX et al (2000) Multiple forms of phosphatase from human brain: isolation and partial characterization of affi-gel blue binding phosphatases. Neurochem Res 25:107–120. doi:10.1023/A:1007547701518
Cheng LY, Wang JZ, Gong CX et al (2001) Multiple forms of phosphatase from human brain: isolation and partial characterization of affi-gel blue nonbinding phosphatase activities. Neurochem Res 26:425–438. doi:10.1023/A:1010963401453
Cho JH, Johnson GV (2003) Glycogen synthase kinase 3beta phosphorylates tau at both primed and unprimed sites. Differential impact on microtubule binding. J Biol Chem 278:187–193. doi:10.1074/jbc.M206236200
Chohan MO, Khatoon S, Iqbal IG, Iqbal K (2006) Involvement of I2PP2A in the abnormal hyperphosphorylation of tau and its reversal by Memantine. FEBS Lett 580:3973–3979. doi:10.1016/j.febslet.2006.06.021
Cohen P (1989) The structure and regulation of protein phosphatases. Annu Rev Biochem 58:453–508. doi:10.1146/annurev.bi.58.070189.002321
Cohen P, Alemany S, Hemmings BA et al (1988) Protein phosphatase-1 and protein phosphatase-2A from rabbit skeletal muscle. Methods Enzymol 159:390–408. doi:10.1016/0076-6879(88)59039-0
Cotman CW, Poon WW, Rissman RA, Blurton-Jones M (2005) The role of caspase cleavage of tau in Alzheimer disease neuropathology. J Neuropathol Exp Neurol 64:104–112
Cripps D, Thomas SN, Jeng Y et al (2006) Alzheimer disease-specific conformation of hyperphosphorylated paired helical filament-Tau is polyubiquitinated through Lys-48, Lys-11, and Lys-6 ubiquitin conjugation. J Biol Chem 281:10825–10838. doi:10.1074/jbc.M512786200
Cruz JC, Tseng HC, Goldman JA, Shih H, Tsai LH (2003) Aberrant Cdk5 activation by p25 triggers pathological events leading to neurodegeneration and neurofibrillary tangles. Neuron 40:471–483. doi:10.1016/S0896-6273(03)00627-5
Delobel P, Lavenir I, Fraser G et al (2008) Analysis of tau phosphorylation and truncation in a mouse model of human tauopathy. Am J Pathol 172:123–131. doi:10.2353/ajpath.2008.070627
Deng Y, Li B, Liu F et al (2008) Regulation between O-GlcNAcylation and phosphorylation of neurofilament-M and their dysregulation in Alzheimer disease. FASEB J 22:138–145. doi:10.1096/fj.07-8309com
Dickey CA, Koren J, Zhang YJ et al (2008) Akt and CHIP coregulate tau degradation through coordinated interactions. Proc Natl Acad Sci USA 105:3622–3627. doi:10.1073/pnas.0709180105
Dickson DW, Farlo J, Davies P et al (1988) Alzheimer’s disease. A double-labeling immunohistochemical study of senile plaques. Am J Pathol 132:86–101
Dickson DW, Crystal HA, Mattiace LA et al (1992) Identification of normal and pathological aging in prospectively studied nondemented elderly humans. Neurobiol Aging 13:179–189. doi:10.1016/0197-4580(92)90027-U
Drewes G, Ebneth A, Preuss U, Mandelkow EM, Mandelkow E (1997) MARK, a novel family of protein kinases that phosphorylate microtubule-associated proteins and trigger microtubule disruption. Cell 89:297–308. doi:10.1016/S0092-8674(00)80208-1
Drewes G, Lichtenberg-Kraag B, Doring F et al (1992) Mitogen activated protein (MAP) kinase transforms tau protein into an Alzheimer-like state. EMBO J 11:2131–2138
Drewes G, Trinczek B, Illenberger S et al (1995) Microtubule-associated protein/microtubule affinity-regulating kinase (p110mark). A novel protein kinase that regulates tau-microtubule interactions and dynamic instability by phosphorylation at the Alzheimer-specific site serine 262. J Biol Chem 270:7679–7688. doi:10.1074/jbc.270.13.7679
Elliott E, Tsvetkov P, Ginzburg I (2007) BAG-1 associates with Hsc70 tau complex and regulates the proteasomal degradation of Tau protein. J Biol Chem 282:37276–37284. doi:10.1074/jbc.M706379200
Engidawork E, Lubec G (2001) Protein expression in Down syndrome brain. Amino Acids 21:331–361. doi:10.1007/s007260170001
Gamblin TC, Chen F, Zambrano A et al (2003) Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer’s disease. Proc Natl Acad Sci USA 100:10032–10037. doi:10.1073/pnas.1630428100
Ghoshal N, Smiley JF, DeMaggio AJ et al (1999) A new molecular link between the fibrillar and granulovacuolar lesions of Alzheimer’s disease. Am J Pathol 155:1163–1172
Goedert M, Spillantini MG, Cairns NJ, Crowther RA (1992) Tau proteins of Alzheimer paired helical filaments: abnormal phosphorylation of all six brain isoforms. Neuron 8:159–168. doi:10.1016/0896-6273(92)90117-V
Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA (1989) Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron 3:519–526. doi:10.1016/0896-6273(89)90210-9
Goedert M, Jakes R, Spillantini MG et al (1996) Assembly of microtubule-associated protein tau into Alzheimer-like filaments induced by sulphated glycosaminoglycans. Nature 383:550–553. doi:10.1038/383550a0
Goldbaum O, Richter-Landsberg C (2004) Proteolytic stress causes heat shock protein induction, tau ubiquitination, and the recruitment of ubiquitin to tau-positive aggregates in oligodendrocytes in culture. J Neurosci 24:5748–5757. doi:10.1523/JNEUROSCI.1307-04.2004
Gong CX, Singh TJ, Grundke-Iqbal I, Iqbal K (1993) Phosphoprotein phosphatase activities in Alzheimer disease brain. J Neurochem 61:921–927. doi:10.1111/j.1471-4159.1993.tb03603.x
Gong CX, Liu F, Grundke-Iqbal I, Iqbal K (2006) Impaired brain glucose metabolism leads to Alzheimer neurofibrillary degeneration through a decrease in tau O-GlcNAcylation. J Alzheimers Dis 9:1–12
Gong CX, Shaikh S, Wang JZ et al (1995) Phosphatase activity toward abnormally phosphorylated tau: decrease in Alzheimer disease brain. J Neurochem 65:732–738
Gong CX, Lidsky T, Wegiel J et al (2000) Phosphorylation of microtubule-associated protein tau is regulated by protein phosphatase 2A in mammalian brain. Implications for neurofibrillary degeneration in Alzheimer’s disease. J Biol Chem 275:5535–5544. doi:10.1074/jbc.275.8.5535
Goode BL, Denis PE, Panda D et al (1997) Functional interactions between the proline-rich and repeat regions of tau enhance microtubule binding and assembly. Mol Biol Cell 8:353–365
Gotz J, Chen F, van Dorpe J, Nitsch RM (2001) Formation of neurofibrillary tangles in P301 l tau transgenic mice induced by Abeta 42 fibrils. Science 293:1491–1495. doi:10.1126/science.1062097
Gray EG, Paula-Barbosa M, Roher A (1987) Alzheimer’s disease: paired helical filaments and cytomembranes. Neuropathol Appl Neurobiol 13:91–110. doi:10.1111/j.1365-2990.1987.tb00174.x
Grundke-Iqbal I, Iqbal K, Tung YC et al (1986) Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA 83:4913–4917. doi:10.1073/pnas.83.13.4913
Grundke-Iqbal I, Iqbal K, Quinlan M et al (1986) Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem 261:6084–6089
Grundke-Iqbal I, Vorbrodt AW, Iqbal K et al (1988) Microtubule-associated polypeptides tau are altered in Alzheimer paired helical filaments. Brain Res 464:43–52
Gunnarsson MD LK, Sudelof J, Basun H, Lannfelt L (2006) Reduction of hyperphosphorylated tau during memantine treatment of Alzheimer’s disease. Alzheimers Dement 2:S63–S64. doi:10.1016/j.jalz.2006.05.229
Hanger DP, Betts JC, Loviny TL, Blackstock WP, Anderton BH (1998) New phosphorylation sites identified in hyperphosphorylated tau (paired helical filament-tau) from Alzheimer’s disease brain using nanoelectrospray mass spectrometry. J Neurochem 71:2465–2476
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297:353–356. doi:10.1126/science.1072994
Hardy JA, Higgins GA (1992) Alzheimer’s disease: the amyloid cascade hypothesis. Science 256:184–185. doi:10.1126/science.1566067
Harrington C, Rickard JE, Horsley D et al (2008) Methylthioninium chloride (MTC) acts as a tau aggregation inhibitor (TAI) in a cellular model and reverses tau pathology in transgenic mouse model of Alzheimer’s disease. Alzheimers Dement 4:120. doi:10.1016/j.jalz.2008.05.259
Hart GW (1997) Dynamic O-linked glycosylation of nuclear and cytoskeletal proteins. Annu Rev Biochem 66:315–335. doi:10.1146/annurev.biochem.66.1.315
Hart GW, Kreppel LK, Comer FI et al (1996) O-GlcNAcylation of key nuclear and cytoskeletal proteins: reciprocity with O-phosphorylation and putative roles in protein multimerization. Glycobiology 6:711–716. doi:10.1093/glycob/6.7.711
Hasegawa M, Crowther RA, Jakes R, Goedert M (1997) Alzheimer-like changes in microtubule-associated protein Tau induced by sulfated glycosaminoglycans. Inhibition of microtubule binding, stimulation of phosphorylation, and filament assembly depend on the degree of sulfation. J Biol Chem 272:33118–33124. doi:10.1074/jbc.272.52.33118
Himmler A, Drechsel D, Kirschner MW, Martin DW Jr (1989) Tau consists of a set of proteins with repeated C-terminal microtubule-binding domains and variable N-terminal domains. Mol Cell Biol 9:1381–1388
Holland AJ, Hon J, Huppert FA, Stevens F, Watson P (1998) Population-based study of the prevalence and presentation of dementia in adults with Down’s syndrome. Br J Psychiatry 172:493–498. doi:10.1192/bjp.172.6.493
Holmes C, Boche D, Wilkinson D et al (2008) Long-term effects of Abeta42 immunisation in Alzheimer’s disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet 372:216–223. doi:10.1016/S0140-6736(08)61075-2
Hong M, Chen DC, Klein PS, Lee VM (1997) Lithium reduces tau phosphorylation by inhibition of glycogen synthase kinase-3. J Biol Chem 272:25326–25332. doi:10.1074/jbc.272.40.25326
Hutton M, Lendon CL, Rizzu P et al (1998) Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 393:702–705. doi:10.1038/31508
Ikegami K, Kimura T, Katsuragi S et al (1996) Immunohistochemical examination of phosphorylated tau in granulovacuolar degeneration granules. Psychiatry Clin Neurosci 50:137–140. doi:10.1111/j.1440-1819.1996.tb01678.x
Iqbal K, Grundke-Iqbal I (2005) Metabolic/signal transduction hypothesis of Alzheimer’s disease and other tauopathies. Acta Neuropathol 109:25–31. doi:10.1007/s00401-004-0951-y
Iqbal K, Zaidi T, Bancher C, Grundke-Iqbal I (1994) Alzheimer paired helical filaments. Restoration of the biological activity by dephosphorylation. FEBS Lett 349:104–108. doi:10.1016/0014-5793(94)00650-4
Iqbal K, Grundke-Iqbal I, Smith AJ et al (1989) Identification and localization of a tau peptide to paired helical filaments of Alzheimer disease. Proc Natl Acad Sci USA 86:5646–5650. doi:10.1073/pnas.86.14.5646
Iqbal K, Grundke-Iqbal I, Zaidi T et al (1986) Defective brain microtubule assembly in Alzheimer’s disease. Lancet 2:421–426. doi:10.1016/S0140-6736(86)92134-3
Iqbal K, Flory M, Khatoon S et al (2005) Subgroups of Alzheimer’s disease based on cerebrospinal fluid molecular markers. Ann Neurol 58:748–757. doi:10.1002/ana.20639
Iqbal K, Alonso Adel C, Chen S et al (2005) Tau pathology in Alzheimer disease and other tauopathies. Biochim Biophys Acta 1739:198–210
Jellinger KA, Attems J (2007) Neurofibrillary tangle-predominant dementia: comparison with classical Alzheimer disease. Acta Neuropathol 113:107–117. doi:10.1007/s00401-006-0156-7
Jicha GA, Berenfeld B, Davies P (1999) Sequence requirements for formation of conformational variants of tau similar to those found in Alzheimer’s disease. J Neurosci Res 55:713–723. doi:10.1002/(SICI)1097-4547(19990315)55:6<713::AID-JNR6>3.0.CO;2-G
Jicha GA, Rockwood JM, Berenfeld B, Hutton M, Davies P (1999) Altered conformation of recombinant frontotemporal dementia-17 mutant tau proteins. Neurosci Lett 260:153–156. doi:10.1016/S0304-3940(98)00980-X
Jicha GA, Lane E, Vincent I et al (1997) A conformation- and phosphorylation-dependent antibody recognizing the paired helical filaments of Alzheimer’s disease. J Neurochem 69:2087–2095
Johnson GV, Hartigan JA (1999) Tau protein in normal and Alzheimer’s disease brain: an update. J Alzheimers Dis 1:329–351
Kampers T, Friedhoff P, Biernat J, Mandelkow EM, Mandelkow E (1996) RNA stimulates aggregation of microtubule-associated protein tau into Alzheimer-like paired helical filaments. FEBS Lett 399:344–349. doi:10.1016/S0014-5793(96)01386-5
Kampers T, Pangalos M, Geerts H, Wiech H, Mandelkow E (1999) Assembly of paired helical filaments from mouse tau: implications for the neurofibrillary pathology in transgenic mouse models for Alzheimer’s disease. FEBS Lett 451:39–44. doi:10.1016/S0014-5793(99)00522-0
Katzman R, Terry R, DeTeresa R et al (1988) Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol 23:138–144. doi:10.1002/ana.410230206
Kentrup H, Becker W, Heukelbach J et al (1996) Dyrk, a dual specificity protein kinase with unique structural features whose activity is dependent on tyrosine residues between subdomains VII and VIII. J Biol Chem 271:3488–3495. doi:10.1074/jbc.271.7.3488
Khatoon S, Grundke-Iqbal I, Iqbal K (1992) Brain levels of microtubule-associated protein tau are elevated in Alzheimer’s disease: a radioimmuno-slot-blot assay for nanograms of the protein. J Neurochem 59:750–753. doi:10.1111/j.1471-4159.1992.tb09432.x
Khatoon S, Grundke-Iqbal I, Iqbal K (1994) Levels of normal and abnormally phosphorylated tau in different cellular and regional compartments of Alzheimer disease and control brains. FEBS Lett 351:80–84. doi:10.1016/0014-5793(94)00829-9
Khatoon S, Grundke-Iqbal I, Iqbal K (1995) Guanosine triphosphate binding to beta-subunit of tubulin in Alzheimer’s disease brain: role of microtubule-associated protein tau. J Neurochem 64:777–787
Kim I, Xu W, Reed JC (2008) Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov 7:1013–1030. doi:10.1038/nrd2755
Kimura R, Kamino K, Yamamoto M et al (2007) The DYRK1A gene, encoded in chromosome 21 Down syndrome critical region, bridges between beta-amyloid production and tau phosphorylation in Alzheimer disease. Hum Mol Genet 16:15–23. doi:10.1093/hmg/ddl437
Kins S, Kurosinski P, Nitsch RM, Gotz J (2003) Activation of the ERK and JNK signaling pathways caused by neuron-specific inhibition of PP2A in transgenic mice. Am J Pathol 163:833–843
Kopke E, Tung YC, Shaikh S et al (1993) Microtubule-associated protein tau Abnormal phosphorylation of a non-paired helical filament pool in Alzheimer disease. J Biol Chem 268:24374–24384
Kovacs GG, Majtenyi K, Spina S et al (2008) White matter tauopathy with globular glial inclusions: a distinct sporadic frontotemporal lobar degeneration. J Neuropathol Exp Neurol 67:963–975. doi:10.1097/NEN.0b013e318187a80f
Kumaran R, Kingsbury A, Coulter I et al (2007) DJ-1 (PARK7) is associated with 3R and 4R tau neuronal and glial inclusions in neurodegenerative disorders. Neurobiol Dis 28:122–132. doi:10.1016/j.nbd.2007.07.012
Kusakawa G, Saito T, Onuki R et al (2000) Calpain-dependent proteolytic cleavage of the p35 cyclin-dependent kinase 5 activator to p25. J Biol Chem 275:17166–17172. doi:10.1074/jbc.M907757199
Lagalwar S, Berry RW, Binder LI (2007) Relation of hippocampal phospho-SAPK/JNK granules in Alzheimer’s disease and tauopathies to granulovacuolar degeneration bodies. Acta Neuropathol 113:63–73. doi:10.1007/s00401-006-0159-4
Lai F, Williams RS (1989) A prospective study of Alzheimer disease in Down syndrome. Arch Neurol 46:849–853
Ledesma MD, Correas I, Avila J, Diaz-Nido J (1992) Implication of brain cdc2 and MAP2 kinases in the phosphorylation of tau protein in Alzheimer’s disease. FEBS Lett 308:218–224. doi:10.1016/0014-5793(92)81278-T
Lee VM, Balin BJ, Otvos L Jr, Trojanowski JQ (1991) A68: a major subunit of paired helical filaments and derivatized forms of normal Tau. Science 251:675–678. doi:10.1126/science.1899488
Levy E, Carman MD, Fernandez-Madrid IJ et al (1990) Mutation of the Alzheimer’s disease amyloid gene in hereditary cerebral hemorrhage, Dutch type. Science 248:1124–1126. doi:10.1126/science.2111584
Lew J, Huang QQ, Qi Z et al (1994) A brain-specific activator of cyclin-dependent kinase 5. Nature 371:423–426. doi:10.1038/371423a0
Lewis J, McGowan E, Rockwood J et al (2000) Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet 25:402–405. doi:10.1038/78078
Li B, Chohan MO, Grundke-Iqbal I, Iqbal K (2007) Disruption of microtubule network by Alzheimer abnormally hyperphosphorylated tau. Acta Neuropathol 113:501–511. doi:10.1007/s00401-007-0207-8
Li L, Sengupta A, Haque N, Grundke-Iqbal I, Iqbal K (2004) Memantine inhibits and reverses the Alzheimer type abnormal hyperphosphorylation of tau and associated neurodegeneration. FEBS Lett 566:261–269. doi:10.1016/j.febslet.2004.04.047
Li M, Guo H, Damuni Z (1995) Purification and characterization of two potent heat-stable protein inhibitors of protein phosphatase 2A from bovine kidney. Biochemistry 34:1988–1996. doi:10.1021/bi00006a020
Li M, Makkinje A, Damuni Z (1996) Molecular identification of I1PP2A, a novel potent heat-stable inhibitor protein of protein phosphatase 2A. Biochemistry 35:6998–7002. doi:10.1021/bi960581y
Li M, Makkinje A, Damuni Z (1996) The myeloid leukemia-associated protein SET is a potent inhibitor of protein phosphatase 2A. J Biol Chem 271:11059–11062. doi:10.1074/jbc.271.19.11059
Li X, Lu F, Wang JZ, Gong CX (2006) Concurrent alterations of O-GlcNAcylation and phosphorylation of tau in mouse brains during fasting. Eur J NeuroSci 23:2078–2086. doi:10.1111/j.1460-9568.2006.04735.x
Liang Z, Liu F, Iqbal K et al (2008) Decrease of protein phosphatase 2A and its association with accumulation and hyperphosphorylation of tau in Down syndrome. J Alzheimers Dis 13:295–302
Liazoghli D, Perreault S, Micheva KD, Desjardins M, Leclerc N (2005) Fragmentation of the Golgi apparatus induced by the overexpression of wild-type and mutant human tau forms in neurons. Am J Pathol 166:1499–1514
Lin WL, Lewis J, Yen SH, Hutton M, Dickson DW (2003) Ultrastructural neuronal pathology in transgenic mice expressing mutant (P301L) human tau. J Neurocytol 32:1091–1105. doi:10.1023/B:NEUR.0000021904.61387.95
Lindwall G, Cole RD (1984) Phosphorylation affects the ability of tau protein to promote microtubule assembly. J Biol Chem 259:5301–5305
Liu F, Iqbal K, Grundke-Iqbal I, Gong CX (2002) Involvement of aberrant glycosylation in phosphorylation of tau by cdk5 and GSK-3beta. FEBS Lett 530:209–214. doi:10.1016/S0014-5793(02)03487-7
Liu F, Grundke-Iqbal I, Iqbal K, Gong CX (2005) Contributions of protein phosphatases PP1, PP2A, PP2B and PP5 to the regulation of tau phosphorylation. Eur J NeuroSci 22:1942–1950. doi:10.1111/j.1460-9568.2005.04391.x
Liu F, Zaidi T, Iqbal K, Grundke-Iqbal I, Gong CX (2002) Aberrant glycosylation modulates phosphorylation of tau by protein kinase A and dephosphorylation of tau by protein phosphatase 2A and 5. Neuroscience 115:829–837. doi:10.1016/S0306-4522(02)00510-9
Liu F, Iqbal K, Grundke-Iqbal I, Hart GW, Gong CX (2004) O-GlcNAcylation regulates phosphorylation of tau: a mechanism involved in Alzheimer’s disease. Proc Natl Acad Sci USA 101:10804–10809. doi:10.1073/pnas.0400348101
Liu F, Zaidi T, Iqbal K et al (2002) Role of glycosylation in hyperphosphorylation of tau in Alzheimer’s disease. FEBS Lett 512:101–106. doi:10.1016/S0014-5793(02)02228-7
Liu F, Liang Z, Shi J et al (2006) PKA modulates GSK-3beta- and cdk5-catalyzed phosphorylation of tau in site- and kinase-specific manners. FEBS Lett 580:6269–6274. doi:10.1016/j.febslet.2006.10.033
Liu F, Liang Z, Wegiel J et al (2008) Overexpression of Dyrk1A contributes to neurofibrillary degeneration in Down syndrome. Faseb J 22:3224–3233
Liu SJ, Zhang JY, Li HL et al (2004) Tau becomes a more favorable substrate for GSK-3 when it is prephosphorylated by PKA in rat brain. J Biol Chem 279:50078–50088. doi:10.1074/jbc.M406109200
Liu YH, Wei W, Yin J et al (2008) Proteasome inhibition increases tau accumulation independent of phosphorylation. Neurobiol Aging (in press)
Lovestone S, Hartley CL, Pearce J, Anderton BH (1996) Phosphorylation of tau by glycogen synthase kinase-3 beta in intact mammalian cells: the effects on the organization and stability of microtubules. Neuroscience 73:1145–1157. doi:10.1016/0306-4522(96)00126-1
Lucas JJ, Hernandez F, Gomez-Ramos P et al (2001) Decreased nuclear beta-catenin, tau hyperphosphorylation and neurodegeneration in GSK-3beta conditional transgenic mice. EMBO J 20:27–39. doi:10.1093/emboj/20.1.27
Luna-Munoz J, Garcia-Sierra F, Falcon V et al (2005) Regional conformational change involving phosphorylation of tau protein at the Thr231, precedes the structural change detected by Alz-50 antibody in Alzheimer’s disease. J Alzheimers Dis 8:29–41
Maeda S, Sahara N, Saito Y et al (2006) Increased levels of granular tau oligomers: an early sign of brain aging and Alzheimer’s disease. Neurosci Res 54:197–201. doi:10.1016/j.neures.2005.11.009
Mah VH, Eskin TA, Kazee AM, Lapham L, Higgins GA (1992) In situ hybridization of calcium/calmodulin dependent protein kinase II and tau mRNAs: species differences and relative preservation in Alzheimer’s disease. Brain Res Mol Brain Res 12:85–94. doi:10.1016/0169-328X(92)90071-I
Mori H, Kondo J, Ihara Y (1987) Ubiquitin is a component of paired helical filaments in Alzheimer’s disease. Science 235:1641–1644. doi:10.1126/science.3029875
Morishima-Kawashima M, Hasegawa M, Takio K et al (1993) Ubiquitin is conjugated with amino-terminally processed tau in paired helical filaments. Neuron 10:1151–1160. doi:10.1016/0896-6273(93)90063-W
Morishima-Kawashima M, Hasegawa M, Takio K et al (1995) Proline-directed and non-proline-directed phosphorylation of PHF-tau. J Biol Chem 270:823–829. doi:10.1074/jbc.270.2.823
Morsch R, Simon W, Coleman PD (1999) Neurons may live for decades with neurofibrillary tangles. J Neuropathol Exp Neurol 58:188–197. doi:10.1097/00005072-199902000-00008
Murphy DB, Borisy GG (1975) Association of high-molecular-weight proteins with microtubules and their role in microtubule assembly in vitro. Proc Natl Acad Sci USA 72:2696–2700. doi:10.1073/pnas.72.7.2696
Nakashima H, Ishihara T, Suguimoto P et al (2005) Chronic lithium treatment decreases tau lesions by promoting ubiquitination in a mouse model of tauopathies. Acta Neuropathol 110:547–556. doi:10.1007/s00401-005-1087-4
Nishi A, Snyder GL, Nairn AC, Greengard P (1999) Role of calcineurin and protein phosphatase-2A in the regulation of DARPP-32 dephosphorylation in neostriatal neurons. J Neurochem 72:2015–2021. doi:10.1046/j.1471-4159.1999.0722015.x
Noble W, Olm V, Takata K et al (2003) Cdk5 is a key factor in tau aggregation and tangle formation in vivo. Neuron 38:555–565. doi:10.1016/S0896-6273(03)00259-9
Novak M, Jakes R, Edwards PC, Milstein C, Wischik CM (1991) Difference between the tau protein of Alzheimer paired helical filament core and normal tau revealed by epitope analysis of monoclonal antibodies 423 and 7.51. Proc Natl Acad Sci USA 88:5837–5841. doi:10.1073/pnas.88.13.5837
Oddo S, Caccamo A, Kitazawa M, Tseng BP, LaFerla FM (2003) Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer’s disease. Neurobiol Aging 24:1063–1070. doi:10.1016/j.neurobiolaging.2003.08.012
Oddo S, Vasilevko V, Caccamo A et al (2006) Reduction of soluble Abeta and tau, but not soluble Abeta alone, ameliorates cognitive decline in transgenic mice with plaques and tangles. J Biol Chem 281:39413–39423. doi:10.1074/jbc.M608485200
Oddo S, Caccamo A, Shepherd JD et al (2003) Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron 39:409–421. doi:10.1016/S0896-6273(03)00434-3
Oliver CJ, Shenolikar S (1998) Physiologic importance of protein phosphatase inhibitors. Front Biosci 3:D961–D972
Oyama F, Cairns NJ, Shimada H et al (1994) Down’s syndrome: up-regulation of beta-amyloid protein precursor and tau mRNAs and their defective coordination. J Neurochem 62:1062–1066
Patzke H, Tsai LH (2002) Calpain-mediated cleavage of the cyclin-dependent kinase-5 activator p39 to p29. J Biol Chem 277:8054–8060. doi:10.1074/jbc.M109645200
Pei JJ, Tanaka T, Tung YC et al (1997) Distribution, levels, and activity of glycogen synthase kinase-3 in the Alzheimer disease brain. J Neuropathol Exp Neurol 56:70–78. doi:10.1097/00005072-199701000-00007
Pei JJ, Grundke-Iqbal I, Iqbal K et al (1998) Accumulation of cyclin-dependent kinase 5 (cdk5) in neurons with early stages of Alzheimer’s disease neurofibrillary degeneration. Brain Res 797:267–277. doi:10.1016/S0006-8993(98)00296-0
Pei JJ, Braak E, Braak H et al (1999) Distribution of active glycogen synthase kinase 3beta (GSK-3beta) in brains staged for Alzheimer disease neurofibrillary changes. J Neuropathol Exp Neurol 58:1010–1019. doi:10.1097/00005072-199909000-00011
Pei JJ, Braak E, Braak H et al (2001) Localization of active forms of C-jun kinase (JNK) and p38 kinase in Alzheimer’s disease brains at different stages of neurofibrillary degeneration. J Alzheimers Dis 3:41–48
Pei JJ, Gong CX, An WL et al (2003) Okadaic-acid-induced inhibition of protein phosphatase 2A produces activation of mitogen-activated protein kinases ERK1/2, MEK1/2, and p70 S6, similar to that in Alzheimer’s disease. Am J Pathol 163:845–858
Pei JJ, An WL, Zhou XW et al (2006) P70 S6 kinase mediates tau phosphorylation and synthesis. FEBS Lett 580:107–114. doi:10.1016/j.febslet.2005.11.059
Perez M, Valpuesta JM, Medina M, Montejo de Garcini E, Avila J (1996) Polymerization of tau into filaments in the presence of heparin: the minimal sequence required for tau-tau interaction. J Neurochem 67:1183–1190
Perez M, Hernandez F, Lim F, Diaz-Nido J, Avila J (2003) Chronic lithium treatment decreases mutant tau protein aggregation in a transgenic mouse model. J Alzheimers Dis 5:301–308
Perry G, Friedman R, Shaw G, Chau V (1987) Ubiquitin is detected in neurofibrillary tangles and senile plaque neurites of Alzheimer disease brains. Proc Natl Acad Sci USA 84:3033–3036. doi:10.1073/pnas.84.9.3033
Perry G, Mulvihill P, Fried VA et al (1989) Immunochemical properties of ubiquitin conjugates in the paired helical filaments of Alzheimer disease. J Neurochem 52:1523–1528. doi:10.1111/j.1471-4159.1989.tb09203.x
Petrucelli L, Dickson D, Kehoe K et al (2004) CHIP and Hsp70 regulate tau ubiquitination, degradation and aggregation. Hum Mol Genet 13:703–714. doi:10.1093/hmg/ddh083
Planel E, Richter KE, Nolan CE et al (2007) Anesthesia leads to tau hyperphosphorylation through inhibition of phosphatase activity by hypothermia. J Neurosci 27:3090–3097. doi:10.1523/JNEUROSCI.4854-06.2007
Poorkaj P, Bird TD, Wijsman E et al (1998) Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann Neurol 43:815–825. doi:10.1002/ana.410430617
Poppek D, Keck S, Ermak G et al (2006) Phosphorylation inhibits turnover of the tau protein by the proteasome: influence of RCAN1 and oxidative stress. Biochem J 400:511–520. doi:10.1042/BJ20060463
Reisberg B, Doody R, Stoffler A et al (2003) Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med 348:1333–1341. doi:10.1056/NEJMoa013128
Roder HM, Eden PA, Ingram VM (1993) Brain protein kinase PK40erk converts TAU into a PHF-like form as found in Alzheimer’s disease. Biochem Biophys Res Commun 193:639–647. doi:10.1006/bbrc.1993.1672
Ruben GC, Iqbal K, Grundke-Iqbal I et al (1991) The microtubule-associated protein tau forms a triple-stranded left-hand helical polymer. J Biol Chem 266:22019–22027
Run X, Liang Z, Zhang L et al (2008) Anesthesia induces phosphorylation of tau. J Alzheimers Dis (in press)
Sahara N, Murayama M, Mizoroki T et al (2005) In vivo evidence of CHIP up-regulation attenuating tau aggregation. J Neurochem 94:1254–1263. doi:10.1111/j.1471-4159.2005.03272.x
Santacruz K, Lewis J, Spires T et al (2005) Tau suppression in a neurodegenerative mouse model improves memory function. Science 309:476–481. doi:10.1126/science.1113694
Schapiro MB, Haxby JV, Grady CL (1992) Nature of mental retardation and dementia in Down syndrome: study with PET, CT, and neuropsychology. Neurobiol Aging 13:723–734. doi:10.1016/0197-4580(92)90096-G
Schmitt H, Gozes I, Littauer UZ (1977) Decrease in levels and rates of synthesis of tubulin and actin in developing rat brain. Brain Res 121:327–342. doi:10.1016/0006-8993(77)90155-X
Schneider A, Biernat J, von Bergen M, Mandelkow E, Mandelkow EM (1999) Phosphorylation that detaches tau protein from microtubules (Ser262, Ser214) also protects it against aggregation into Alzheimer paired helical filaments. Biochemistry 38:3549–3558. doi:10.1021/bi981874p
Scott CW, Spreen RC, Herman JL et al (1993) Phosphorylation of recombinant tau by cAMP-dependent protein kinase Identification of phosphorylation sites and effect on microtubule assembly. J Biol Chem 268:1166–1173
Sengupta A, Novak M, Grundke-Iqbal I, Iqbal K (2006) Regulation of phosphorylation of tau by cyclin-dependent kinase 5 and glycogen synthase kinase-3 at substrate level. FEBS Lett 580:5925–5933
Sengupta A, Wu Q, Grundke-Iqbal I, Iqbal K, Singh TJ (1997) Potentiation of GSK-3-catalyzed Alzheimer-like phosphorylation of human tau by cdk5. Mol Cell Biochem 167:99–105. doi:10.1023/A:1006883924775
Shapiro BL (1999) The Down syndrome critical region. J Neural Transm Suppl 57:41–60
Shi J, Zhang T, Zhou C et al (2008) Increased dosage of Dyrk1A alters alternative splicing factor (ASF)-regulated alternative splicing of tau in Down syndrome. J Biol Chem 283:28660–28669. doi:10.1074/jbc.M802645200
Shioi J, Georgakopoulos A, Mehta P et al (2007) FAD mutants unable to increase neurotoxic Abeta 42 suggest that mutation effects on neurodegeneration may be independent of effects on Abeta. J Neurochem 101:674–681. doi:10.1111/j.1471-4159.2006.04391.x
Sibille N, Sillen A, Leroy A et al (2006) Structural impact of heparin binding to full-length Tau as studied by NMR spectroscopy. Biochemistry 45:12560–12572. doi:10.1021/bi060964o
Singh TJ, Grundke-Iqbal I, Iqbal K (1995) Phosphorylation of tau protein by casein kinase-1 converts it to an abnormal Alzheimer-like state. J Neurochem 64:1420–1423
Singh TJ, Grundke-Iqbal I, McDonald B, Iqbal K (1994) Comparison of the phosphorylation of microtubule-associated protein tau by non-proline dependent protein kinases. Mol Cell Biochem 131:181–189. doi:10.1007/BF00925955
Singh TJ, Wang JZ, Novak M et al (1996) Calcium/calmodulin-dependent protein kinase II phosphorylates tau at Ser-262 but only partially inhibits its binding to microtubules. FEBS Lett 387:145–148. doi:10.1016/0014-5793(96)00485-1
Sironi JJ, Yen SH, Gondal JA et al (1998) Ser-262 in human recombinant tau protein is a markedly more favorable site for phosphorylation by CaMKII than PKA or PhK. FEBS Lett 436:471–475. doi:10.1016/S0014-5793(98)01185-5
Sloboda RD, Rudolph SA, Rosenbaum JL, Greengard P (1975) Cyclic AMP-dependent endogenous phosphorylation of a microtubule-associated protein. Proc Natl Acad Sci USA 72:177–181. doi:10.1073/pnas.72.1.177
Spillantini MG, Murrell JR, Goedert M et al (1998) Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc Natl Acad Sci USA 95:7737–7741. doi:10.1073/pnas.95.13.7737
Spittaels K, Van den Haute C, Van Dorpe J et al (2000) Glycogen synthase kinase-3beta phosphorylates protein tau and rescues the axonopathy in the central nervous system of human four-repeat tau transgenic mice. J Biol Chem 275:41340–41349. doi:10.1074/jbc.M006219200
Stambolic V, Ruel L, Woodgett JR (1996) Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol 6:1664–1668. doi:10.1016/S0960-9822(02)70790-2
Steiner B, Mandelkow EM, Biernat J et al (1990) Phosphorylation of microtubule-associated protein tau: identification of the site for Ca2(+)-calmodulin dependent kinase and relationship with tau phosphorylation in Alzheimer tangles. EMBO J 9:3539–3544
Su B, Wang X, Drew KL et al (2008) Physiological regulation of tau phosphorylation during hibernation. J Neurochem (E-pub ahead of print)
Tanaka T, Zhong J, Iqbal K, Trenkner E, Grundke-Iqbal I (1998) The regulation of phosphorylation of tau in SY5Y neuroblastoma cells: the role of protein phosphatases. FEBS Lett 426:248–254. doi:10.1016/S0014-5793(98)00346-9
Tanimukai H, Grundke-Iqbal I, Iqbal K (2005) Up-regulation of inhibitors of protein phosphatase-2A in Alzheimer’s disease. Am J Pathol 166:1761–1771
Tatebayashi Y, Haque N, Tung YC, Iqbal K, Grundke-Iqbal I (2004) Role of tau phosphorylation by glycogen synthase kinase-3beta in the regulation of organelle transport. J Cell Sci 117:1653–1663. doi:10.1242/jcs.01018
Tomlinson BE, Blessed G, Roth M (1970) Observations on the brains of demented old people. J Neurol Sci 11:205–242. doi:10.1016/0022-510X(70)90063-8
Tsai LH, Takahashi T, Caviness VS Jr, Harlow E (1993) Activity and expression pattern of cyclin-dependent kinase 5 in the embryonic mouse nervous system. Development 119:1029–1040
Tsujio I, Zaidi T, Xu J et al (2005) Inhibitors of protein phosphatase-2A from human brain structures, immunocytological localization and activities towards dephosphorylation of the Alzheimer type hyperphosphorylated tau. FEBS Lett 579:363–372. doi:10.1016/j.febslet.2004.11.097
Ulitzur N, Rancano C, Pfeffer SR (1997) Biochemical characterization of mapmodulin, a protein that binds microtubule-associated proteins. J Biol Chem 272:30577–30582. doi:10.1074/jbc.272.48.30577
van Leeuwen FW, Hol EM, Fischer DF (2006) Frameshift proteins in Alzheimer’s disease and in other conformational disorders: time for the ubiquitin-proteasome system. J Alzheimers Dis 9:319–325
Vandebroek T, Terwel D, Vanhelmont T et al (2006) Microtubule binding and clustering of human Tau-4R and Tau-P301L proteins isolated from yeast deficient in orthologues of glycogen synthase kinase-3beta or cdk5. J Biol Chem 281:25388–25397. doi:10.1074/jbc.M602792200
Vandebroek T, Vanhelmont T, Terwel D et al (2005) Identification and isolation of a hyperphosphorylated, conformationally changed intermediate of human protein tau expressed in yeast. Biochemistry 44:11466–11475. doi:10.1021/bi0506775
von Bergen M, Friedhoff P, Biernat J et al (2000) Assembly of tau protein into Alzheimer paired helical filaments depends on a local sequence motif ((306)VQIVYK(311)) forming beta structure. Proc Natl Acad Sci USA 97:5129–5134. doi:10.1073/pnas.97.10.5129
von Lindern M, van Baal S, Wiegant J et al (1992) Can, a putative oncogene associated with myeloid leukemogenesis, may be activated by fusion of its 3′ half to different genes: characterization of the set gene. Mol Cell Biol 12:3346–3355
Wagner U, Utton M, Gallo JM, Miller CC (1996) Cellular phosphorylation of tau by GSK-3 beta influences tau binding to microtubules and microtubule organisation. J Cell Sci 109(Pt 6):1537–1543
Walaas SI, Greengard P (1991) Protein phosphorylation and neuronal function. Pharmacol Rev 43:299–349
Wang JZ, Grundke-Iqbal I, Iqbal K (1996) Restoration of biological activity of Alzheimer abnormally phosphorylated tau by dephosphorylation with protein phosphatase-2A, -2B and -1. Brain Res Mol Brain Res 38:200–208. doi:10.1016/0169-328X(95)00316-K
Wang JZ, Grundke-Iqbal I, Iqbal K (1996) Glycosylation of microtubule-associated protein tau: an abnormal posttranslational modification in Alzheimer’s disease. Nat Med 2:871–875. doi:10.1038/nm0896-871
Wang JZ, Grundke-Iqbal I, Iqbal K (2007) Kinases and phosphatases and tau sites involved in Alzheimer neurofibrillary degeneration. Eur J NeuroSci 25:59–68
Wang JZ, Gong CX, Zaidi T, Grundke-Iqbal I, Iqbal K (1995) Dephosphorylation of Alzheimer paired helical filaments by protein phosphatase-2A and -2B. J Biol Chem 270:4854–4860. doi:10.1074/jbc.270.9.4854
Wang JZ, Wu Q, Smith A, Grundke-Iqbal I, Iqbal K (1998) Tau is phosphorylated by GSK-3 at several sites found in Alzheimer disease and its biological activity markedly inhibited only after it is prephosphorylated by A-kinase. FEBS Lett 436:28–34. doi:10.1016/S0014-5793(98)01090-4
Wang X, Blanchard J, Li B, Fan G, Liu F, Linden RM, Clement N, Kohlbrenner E, Radu A, Grundke-Iqbal I, Iqbal K (2008) Generation of a non-transgenic rat model of Alzheimer-like abnormal hyperphosphorylation of tau. Abstract presented at the 11th international conference on Alzheimer's disease (ICAD), Chicago, IL, USA, 26–31 July, 2008
Wegiel J, Wisniewski HM, Dziewiatkowski J, Popovitch ER, Tarnawski M (1996) Differential susceptibility to neurofibrillary pathology among patients with Down syndrome. Dementia 7:135–141. doi:10.1159/000106868
Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW (1975) A protein factor essential for microtubule assembly. Proc Natl Acad Sci USA 72:1858–1862. doi:10.1073/pnas.72.5.1858
Wischik CM, Bentham P, Wischik DJ, Seng KM (2008) Tau aggregation inhibitor (TAI) therapy with rember arrests disease progression in mild and moderate Alzheimer’s disease over 50 weeks. Alzheimers Dement 4:167. doi:10.1016/j.jalz.2008.05.438
Wisniewski KE, Wisniewski HM, Wen GY (1985) Occurrence of neuropathological changes and dementia of Alzheimer’s disease in Down’s syndrome. Ann Neurol 17:278–282. doi:10.1002/ana.410170310
Wittmann CW, Wszolek MF, Shulman JM et al (2001) Tauopathy in Drosophila: neurodegeneration without neurofibrillary tangles. Science 293:711–714. doi:10.1126/science.1062382
Woodgett JR (1990) Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J 9:2431–2438
Woods YL, Cohen P, Becker W et al (2001) The kinase DYRK phosphorylates protein-synthesis initiation factor eIF2Bepsilon at Ser539 and the microtubule-associated protein tau at Thr212: potential role for DYRK as a glycogen synthase kinase 3-priming kinase. Biochem J 355:609–615
Yang L, Ksiezak-Reding H (1998) Ubiquitin immunoreactivity of paired helical filaments differs in Alzheimer’s disease and corticobasal degeneration. Acta Neuropathol 96:520–526. doi:10.1007/s004010050928
Yang LS, Ksiezak-Reding H (1995) Calpain-induced proteolysis of normal human tau and tau associated with paired helical filaments. Eur J Biochem 233:9–17. doi:10.1111/j.1432-1033.1995.009_1.x
Yoshida M (2006) Cellular tau pathology and immunohistochemical study of tau isoforms in sporadic tauopathies. Neuropathology 26:457–470. doi:10.1111/j.1440-1789.2006.00743.x
Yoshiyama Y, Higuchi M, Zhang B et al (2007) Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 53:337–351. doi:10.1016/j.neuron.2007.01.010
Yuzwa SA, Macauley MS, Heinonen JE et al (2008) A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. Nat Chem Biol 4:483–490
Zilka N, Filipcik P, Koson P et al (2006) Truncated tau from sporadic Alzheimer’s disease suffices to drive neurofibrillary degeneration in vivo. FEBS Lett 580:3582–3588. doi:10.1016/j.febslet.2006.05.029
Acknowledgments
We are grateful to Janet Murphy for secretarial assistance. Studies in our laboratories were supported in part by the New York State Office of Mental Retardation and Developmental Disabilities and NIH grants AG019158, AG028538, and AG27429, and Alzheimer’s Association (Chicago, IL) grants IIRG-00-2002, IIRG-05-13095, and NIRG-08-91126.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iqbal, K., Liu, F., Gong, CX. et al. Mechanisms of tau-induced neurodegeneration. Acta Neuropathol 118, 53–69 (2009). https://doi.org/10.1007/s00401-009-0486-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-009-0486-3