Abstract
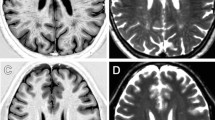
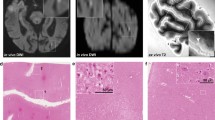
Neuroimaging with iron-sensitive MR sequences [gradient echo T2* and susceptibility-weighted imaging (SWI)] identifies small signal voids that are suspected brain microbleeds. Though the clinical significance of these lesions remains uncertain, their distribution and prevalence correlates with cerebral amyloid angiopathy (CAA), hypertension, smoking, and cognitive deficits. Investigation of the pathologies that produce signal voids is necessary to properly interpret these imaging findings. We conducted a systematic correlation of SWI-identified hypointensities to tissue pathology in postmortem brains with Alzheimer’s disease (AD) and varying degrees of CAA. Autopsied brains from eight AD patients, six of which showed advanced CAA, were imaged at 3T; foci corresponding to hypointensities were identified and studied histologically. A variety of lesions was detected; the most common lesions were acute microhemorrhage, hemosiderin residua of old hemorrhages, and small lacunes ringed by hemosiderin. In lesions where the bleeding vessel could be identified, β-amyloid immunohistochemistry confirmed the presence of β-amyloid in the vessel wall. Significant cellular apoptosis was noted in the perifocal region of recent bleeds along with heme oxygenase 1 activity and late complement activation. Acutely extravasated blood and hemosiderin were noted to migrate through enlarged Virchow–Robin spaces propagating an inflammatory reaction along the local microvasculature; a mechanism that may contribute to the formation of lacunar infarcts. Correlation of imaging findings to tissue pathology in our cases indicates that a variety of CAA-related pathologies produce MR-identified signal voids and further supports the use of SWI as a biomarker for this disease.






Similar content being viewed by others
Abbreviations
- GRE-T2*:
-
Gradient echo T2*
- SWI:
-
Susceptibility-weighted imaging
- MR:
-
Magnetic resonance
- BMB:
-
Brain microbleed
- CAA:
-
Cerebral amyloid angiopathy
- AD:
-
Alzheimer’s disease
- HO-1:
-
Heme oxygenase 1
References
Aliev G, Smith MA, Seyidov D et al (2002) The role of oxidative stress in the pathophysiology of cerebrovascular lesions in Alzheimer’s disease. Brain Pathol 12:21–35
Anders K, Wang Z, Kornfeld M et al (1997) Giant cell arteritis in association with cerebral amyloid angiopathy: immunohistochemical and molecular studies. Hum Pathol 89:1237–1246
Atlas SW, Mark AS, Grossman RI, Gomori JM (1988) Intracranial hemorrhage: gradient-echo MR imaging at 1.5 T. Comparison with spin-echo imaging and clinical applications. Radiology 168:803–807
Blamire A, Rowe J, Styles P, McDonald B (1999) Optimising imaging parameters for post mortem MR imaging of the human brain. Acta Radiol 40:593–597
Bush V, Moyer T, Batts K, Parisi J (1995) Essential and toxic element concentrations in fresh and formalin-fixed human autopsy tissues. Clin Chem 41(2):284–294
Cadmen E, Puttfarcken P (1997) Beta-amyloid peptides initiate the complement cascade without producing a comparable effect on the terminal pathway in vitro. Exp Neurol 146:388–394
Carvlin M, Asato R, Hackney D, Kassab E, Joseph P (1989) High-resolution MR of the spinal cord in humans and rats. Am J Neuroradiol 10:13–17
Cordonnier C, Al-Shahi Salman R, Wardlaw J (2007) Spontaneous brain microbleeds: systematic review, subgroup analyses and standards for study design and reporting. Brain 130:1988–2003
Cordonnier C, Potter G, Jackson C, Doubal F, Sodlow C, Wardlaw J, Al-Shahi Salman R (2008) Improving interrater agreement about brain microbleeds: development of the Brain Observer MicroBleed Scale. Stroke 40:94–99
Cordonnier C, van der Flier WM, Sluimer JD, Leys D, Barkhof F, Scheltens P (2006) Prevalence and severity of microbleeds in a memory clinic setting. Neurology 66:1356–1360
Cullen KM, Kocsi Z, Stone J (2005) Pericapillary haem-rich deposits: evidence for microhaemorrhages in aging human cerebral cortex. J Cereb Blood Flow Metab 25:1656–1667
Davis J, Cribbs DH, Cotman CW, Van Nostrand WE (1999) Pathogenic amyloid-beta protein induces apoptosis in cultured human cerebrovascular smooth muscle cells. Amyloid 6:157–164
Ellis R, Olichney J, Thal L et al (1996) Cerebral amyloid angiopathy in the brains of patients with Alzheimer’s disease: the CERAD experience, Part XV. Neurology 46:1592–1596
Fazekas F, Kleinert R, Roob G et al (1999) Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy-related microbleeds. AJNR Am J Neuroradiol 20:637–642
Fisher C (1991) Lacunar infarcts—a review. Cerebrovasc Dis 1:311–320
Greenberg S, Nandigam R, Delgado P et al (2009) Microbleeds versus macrobleeds: evidence for distinct entities. Stroke 40:2382–2386
Greenberg S, Vonsattel JP (1997) Diagnosis of cerebral amyloid angiopathy. Stroke 28:1418–1422
Haacke EM, Cheng NY, House MJ et al (2005) Imaging iron stores in the brain using magnetic resonance imaging. Magn Reson Imaging 23:1–25
Haacke EM, DelProposto ZS, Chaturvedi S et al (2007) Imaging cerebral amyloid angiopathy with susceptibility-weighted imaging. AJNR Am J Neuroradiol 28:316–317
Haacke EM, Xu Y, Cheng YC, Reichenbach JR (2004) Susceptibility weighted imaging (SWI). Magn Reson Med 52:612–618
Igase M, Tabara Y, Igase K et al (2009) Asymptomatic cerebral microbleeds seen in healthy subjects have a strong association with asymptomatic lacunar infarction. Circ J 73:530–533
Jellinger KA, Lauda F, Attems J (2007) Sporadic cerebral amyloid angiopathy is not a frequent cause of spontaneous brain hemorrhage. Eur J Neurol 14:923–928
Kirsch W, McAuley G, Holshouser B et al (2009) Serial susceptibility weighted MRI measures brain iron and microbleeds in dementia. J Alzheimer’s Dis 17:599–609
Knudsen KA, Rosand J, Karluk D, Greenberg SM (2001) Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology 56:537–539
Koennecke HC (2006) Cerebral microbleeds on MRI: prevalence, associations, and potential clinical implications. Neurology 66:165–171
Nicholl R, Balasubramanian V, Urguhart D, Sellathurai N, Rutherford M (2007) Postmortem brain MRI with selective tissue biopsy as an adjunct to autopsy following neonatal encephalopathy. Eur J Paediatr Neurol 11:167–174
Rosand J, Muzikansky A, Kumar A et al (2005) Spatial clustering of hemorrhages in probable cerebral amyloid angiopathy. Ann Neurol 58:459–462
Pfefferbaum A, Sullivan E, Adalsteinssom E, Garrick T, Harper C (2004) Postmortem MR imaging of formalin-fixed human brain. Neuroimage 21:1585–1595
Pollock H, Hutchings M, Weller RO, Zhang ET (1997) Perivascular spaces in the basal ganglia of the human brain: their relationship to lacunes. J Anat 191:337–347
Roher A, Kuo Y, Esh C et al (2003) Cortical and leptomeningeal cerebrovascular amyloid and white matter pathology in Alzheimer’s disease. Mol Med 9:112–122
Schley D, Carare-Nnadi R, Please C, Perry V, Weller R (2006) Mechanisms to explain the reverse perivascular transport of solutes out of the brain. J Theor Biol 238:962–974
Schmierer K, Wheeler-Kingshott C, Boulby P et al (2007) Diffusion tensor imaging of post mortem multiple sclerosis brain. Neuroimage 35:467–477
Sehgal V, Delproposto Z, Haacke EM et al (2005) Clinical applications of neuroimaging with susceptibility-weighted imaging. J Magn Reson Imaging 22:439–450
Soontornniyomkij V, Lynch M, Mermash S et al (2009) Cerebral microinfarcts associated with severe cerebral beta-amyloid angiopathy. Brain Path (in press)
Sveinbjornsdottir S, Sigurdsson S, Aspelund T et al (2008) Cerebral microbleeds in the population based AGES-Reykjavik study: prevalence and location. J Neurol Neurosurg Psychiatry 79:1002–1006
Tanaka A, Ueno Y, Nakayama Y, Takano K, Takebayashi S (1999) Small chronic microhemorrhages and ischemic lesion in association with spontaneous intracerebral hematomas. Stroke 30:1637–1642
Tatsumi S, Shinohara M, Yamamoto T (2008) Direct comparison of histology of microbleed with postmortem MR images: a case report. Cerebrovasc Dis 26:142–146
Terai K, Walker D, McGeer E, McGeer P (1997) Neurons express proteins of the classical complement pathway in Alzheimer disease. Brain Res 769:385–390
Tong KA, Ashwal S, Holshouser BA et al (2003) Hemorrhagic shearing lesions in children and adolescents with posttraumatic diffuse axonal injury: improved detection and initial results. Radiology 227:332–339
Tong KA, Ashwal S, Holshouser BA et al (2004) Diffuse axonal injury in children: clinical correlation with hemorrhagic lesions. Ann Neurol 56:36–50
Viswanathan A, Chabriat H (2006) Cerebral microhemorrhage. Stroke 37:550–555
Walker DA, Broderick DF, Kotsenas AL, Rubino FA (2004) Routine use of gradient-echo MRI to screen for cerebral amyloid angiopathy in elderly patients. AJR Am J Roentgenol 182:1547–1550
Walker D, Dalsing-Hernandez J, Lue L (2008) Human postmortem brain-derived cerebrovascular smooth muscle cells express all genes of the classical complement pathway: a potential mechanism for vascular damage in cerebral amyloid angiopathy and Alzheimer’s disease. Microvasc Res 75:411–419
Wardlaw J, Dennis M, Warlow, Sandercock P (2001) Imaging appearance of the symptomatic perforating artery in patients with lacunar infarction: 1 occlusion or other vascular pathology. Ann Neurol 50:208–15
Yakushiji Y, Nishiyama M, Yakushiji S et al (2008) Brain microbleeds and global cognitive function in adults without neurological disorder. Stroke 39:3323–3328
Yasojima K, Schwab C, McGeer E, McGeer P (1999) Up-regulated production and activation of the complement system in Alzheimer’s disease brain. Am J Pathol 154:927–936
Acknowledgments
This research was funded by the National Institutes of Health (AG20948). Harry V. Vinters is supported in part by P01 AG12435, P50 AG16570 and the Daljit S. and Elaine Sarkaria Chair in Diagnostic Medicine. E. Mark Haacke is a consultant to Siemens Corporation. None of the other authors have real or potential conflicts of interest related to this work. We thank Zachary Taylor who was an undergraduate summer researcher in our lab, as well as Cindy Dickson, April Dickson and Jackie Knecht for administrative assistance and for editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schrag, M., McAuley, G., Pomakian, J. et al. Correlation of hypointensities in susceptibility-weighted images to tissue histology in dementia patients with cerebral amyloid angiopathy: a postmortem MRI study. Acta Neuropathol 119, 291–302 (2010). https://doi.org/10.1007/s00401-009-0615-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-009-0615-z