Abstract
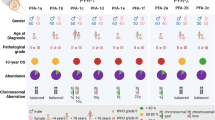
Of nine ependymoma molecular groups detected by DNA methylation profiling, the posterior fossa type A (PFA) is most prevalent. We used DNA methylation profiling to look for further molecular heterogeneity among 675 PFA ependymomas. Two major subgroups, PFA-1 and PFA-2, and nine minor subtypes were discovered. Transcriptome profiling suggested a distinct histogenesis for PFA-1 and PFA-2, but their clinical parameters were similar. In contrast, PFA subtypes differed with respect to age at diagnosis, gender ratio, outcome, and frequencies of genetic alterations. One subtype, PFA-1c, was enriched for 1q gain and had a relatively poor outcome, while patients with PFA-2c ependymomas showed an overall survival at 5 years of > 90%. Unlike other ependymomas, PFA-2c tumors express high levels of OTX2, a potential biomarker for this ependymoma subtype with a good prognosis. We also discovered recurrent mutations among PFA ependymomas. H3 K27M mutations were present in 4.2%, occurring only in PFA-1 tumors, and missense mutations in an uncharacterized gene, CXorf67, were found in 9.4% of PFA ependymomas, but not in other groups. We detected high levels of wildtype or mutant CXorf67 expression in all PFA subtypes except PFA-1f, which is enriched for H3 K27M mutations. PFA ependymomas are characterized by lack of H3 K27 trimethylation (H3 K27-me3), and we tested the hypothesis that CXorf67 binds to PRC2 and can modulate levels of H3 K27-me3. Immunoprecipitation/mass spectrometry detected EZH2, SUZ12, and EED, core components of the PRC2 complex, bound to CXorf67 in the Daoy cell line, which shows high levels of CXorf67 and no expression of H3 K27-me3. Enforced reduction of CXorf67 in Daoy cells restored H3 K27-me3 levels, while enforced expression of CXorf67 in HEK293T and neural stem cells reduced H3 K27-me3 levels. Our data suggest that heterogeneity among PFA ependymomas could have clinicopathologic utility and that CXorf67 may have a functional role in these tumors.







Similar content being viewed by others
References
Alexander T, Nolte C, Krumlauf R (2009) Hox genes and segmentation of the hindbrain and axial skeleton. Annu Rev Cell Dev Biol 25:431–456. https://doi.org/10.1146/annurev.cellbio.042308.113423
Bayliss J, Mukherjee P, Lu C, Jain SU, Chung C, Martinez D et al (2016) Lowered H3K27me3 and DNA hypomethylation define poorly prognostic pediatric posterior fossa ependymomas. Sci Transl Med 8:366ra161. https://doi.org/10.1126/scitranslmed.aah6904
Bender S, Tang Y, Lindroth AM, Hovestadt V, Jones DT, Kool M et al (2013) Reduced H3K27me3 and DNA hypomethylation are major drivers of gene expression in K27M mutant pediatric high-grade gliomas. Cancer Cell 24:660–672. https://doi.org/10.1016/j.ccr.2013.10.006
Bouffet E, Tabori U, Huang A, Bartels U (2009) Ependymoma: lessons from the past, prospects for the future. Childs Nerv Syst 25:1383–1384. https://doi.org/10.1007/s00381-009-0915-6
Boulay G, Awad ME, Riggi N, Archer TC, Iyer S, Boonseng WE et al (2017) OTX2 activity at distal regulatory elements shapes the chromatin landscape of group 3 medulloblastoma. Cancer Discov 7:288–301. https://doi.org/10.1158/2159-8290.CD-16-0844
Capper D, Jones DTW, Sill M, Hovestadt V, Schrimpf D, Sturm D et al (2018) DNA methylation-based classification of central nervous system tumours. Nature 555:469–474. https://doi.org/10.1038/nature26000
Carter M, Nicholson J, Ross F, Crolla J, Allibone R, Balaji V et al (2002) Genetic abnormalities detected in ependymomas by comparative genomic hybridisation. Br J Cancer 86:929–939
Dewaele B, Przybyl J, Quattrone A, Finalet Ferreiro J, Vanspauwen V, Geerdens E et al (2014) Identification of a novel, recurrent MBTD1-CXorf67 fusion in low-grade endometrial stromal sarcoma. Int J Cancer 134:1112–1122. https://doi.org/10.1002/ijc.28440
Di Giovannantonio LG, Di Salvio M, Omodei D, Prakash N, Wurst W, Pierani A et al (2014) Otx2 cell-autonomously determines dorsal mesencephalon versus cerebellum fate independently of isthmic organizing activity. Development 141:377–388. https://doi.org/10.1242/dev.102954
Dosztanyi Z, Csizmok V, Tompa P, Simon I (2005) IUPred: web server for the prediction of intrinsically unstructured regions of proteins based on estimated energy content. Bioinformatics 21:3433–3434. https://doi.org/10.1093/bioinformatics/bti541
Dosztanyi Z, Meszaros B, Simon I (2009) ANCHOR: web server for predicting protein binding regions in disordered proteins. Bioinformatics 25:2745–2746. https://doi.org/10.1093/bioinformatics/btp518
Dyer S, Prebble E, Davison V, Davies P, Ramani P, Ellison D et al (2002) Genomic imbalances in pediatric intracranial ependymomas define clinically relevant groups. Am J Pathol 161:2133–2141
Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO et al (2013) Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 6:l1. https://doi.org/10.1126/scisignal.2004088
Gessi M, Capper D, Sahm F, Huang K, von Deimling A, Tippelt S et al (2016) Evidence of H3 K27M mutations in posterior fossa ependymomas. Acta Neuropathol 132:635–637. https://doi.org/10.1007/s00401-016-1608-3
Gessi M, Gielen GH, Dreschmann V, Waha A, Pietsch T (2015) High frequency of H3F3A (K27 M) mutations characterizes pediatric and adult high-grade gliomas of the spinal cord. Acta Neuropathol 130:435–437. https://doi.org/10.1007/s00401-015-1463-7
Godfraind C, Kaczmarska JM, Kocak M, Dalton J, Wright KD, Sanford RA et al (2012) Distinct disease-risk groups in pediatric supratentorial and posterior fossa ependymomas. Acta Neuropathol 124:247–257. https://doi.org/10.1007/s00401-012-0981-9
Hirate Y, Okamoto H (2006) Canopy1, a novel regulator of FGF signaling around the midbrain–hindbrain boundary in zebrafish. Curr Biol 16:421–427. https://doi.org/10.1016/j.cub.2006.01.055
Hoffman LM, Donson AM, Nakachi I, Griesinger AM, Birks DK, Amani V et al (2014) Molecular sub-group-specific immunophenotypic changes are associated with outcome in recurrent posterior fossa ependymoma. Acta Neuropathol 127:731–745. https://doi.org/10.1007/s00401-013-1212-8
Huang YJ, Acton TB, Montelione GT (2014) DisMeta: a meta server for construct design and optimization. Methods Mol Biol 1091:3–16. https://doi.org/10.1007/978-1-62703-691-7_1
Huber W, Carey VJ, Gentleman R, Anders S, Carlson M, Carvalho BS et al (2015) Orchestrating high-throughput genomic analysis with bioconductor. Nat Methods 12:115–121. https://doi.org/10.1038/nmeth.3252
Johann PD, Erkek S, Zapatka M, Kerl K, Buchhalter I, Hovestadt V et al (2016) Atypical teratoid/rhabdoid tumors are comprised of three epigenetic subgroups with distinct enhancer landscapes. Cancer Cell 29:379–393. https://doi.org/10.1016/j.ccell.2016.02.001
Joyon N, Tauziede-Espariat A, Alentorn A, Giry M, Castel D, Capelle L et al (2017) K27M mutation in H3F3A in ganglioglioma grade I with spontaneous malignant transformation extends the histopathological spectrum of the histone H3 oncogenic pathway. Neuropathol Appl Neurobiol 43:271–276. https://doi.org/10.1111/nan.12329
Kilday JP, Mitra B, Domerg C, Ward J, Andreiuolo F, Osteso-Ibanez T et al (2012) Copy number gain of 1q25 predicts poor progression-free survival for pediatric intracranial ependymomas and enables patient risk stratification: a prospective European clinical trial cohort analysis on behalf of the Children’s Cancer Leukaemia Group (CCLG), Societe Francaise d’Oncologie Pediatrique (SFOP), and International Society for Pediatric Oncology (SIOP). Clin Cancer Res 18:2001–2011. https://doi.org/10.1158/1078-0432.CCR-11-2489
Korshunov A, Witt H, Hielscher T, Benner A, Remke M, Ryzhova M et al (2010) Molecular staging of intracranial ependymoma in children and adults. J Clin Oncol 28:3182–3190. https://doi.org/10.1200/JCO.2009.27.3359
Ma X, Wang J, Wang J, Ma CX, Gao X, Patriub V et al (2017) The JAZF1-SUZ12 fusion protein disrupts PRC2 complexes and impairs chromatin repression during human endometrial stromal tumorogenesis. Oncotarget 8:4062–4078. https://doi.org/10.18632/oncotarget.13270
Mack SC, Pajtler KW, Chavez L, Okonechnikov K, Bertrand KC, Wang X et al (2018) Therapeutic targeting of ependymoma as informed by oncogenic enhancer profiling. Nature 553:101–105. https://doi.org/10.1038/nature25169
Mack SC, Witt H, Piro RM, Gu L, Zuyderduyn S, Stutz AM et al (2014) Epigenomic alterations define lethal CIMP-positive ependymomas of infancy. Nature 506:445–450. https://doi.org/10.1038/nature13108
McGuire CS, Sainani KL, Fisher PG (2009) Incidence patterns for ependymoma: a surveillance, epidemiology, and end results study. J Neurosurg 110:725–729. https://doi.org/10.3171/2008.9.JNS08117
Mendrzyk F, Korshunov A, Benner A, Toedt G, Pfister S, Radlwimmer B et al (2006) Identification of gains on 1q and epidermal growth factor receptor overexpression as independent prognostic markers in intracranial ependymoma. Clin Cancer Res 12:2070–2079. https://doi.org/10.1158/1078-0432.CCR-05-2363
Micci F, Panagopoulos I, Bjerkehagen B, Heim S (2006) Consistent rearrangement of chromosomal band 6p21 with generation of fusion genes JAZF1/PHF1 and EPC1/PHF1 in endometrial stromal sarcoma. Cancer Res 66:107–112. https://doi.org/10.1158/0008-5472.CAN-05-2485
Pajtler KW, Mack SC, Ramaswamy V, Smith CA, Witt H, Smith A et al (2017) The current consensus on the clinical management of intracranial ependymoma and its distinct molecular variants. Acta Neuropathol 133:5–12. https://doi.org/10.1007/s00401-016-1643-0
Pajtler KW, Witt H, Sill M, Jones DT, Hovestadt V, Kratochwil F et al (2015) Molecular classification of ependymal tumors across all CNS compartments, histopathological grades, and age groups. Cancer Cell 27:728–743. https://doi.org/10.1016/j.ccell.2015.04.002
Panwalkar P, Clark J, Ramaswamy V, Hawes D, Yang F, Dunham C et al (2017) Immunohistochemical analysis of H3K27me3 demonstrates global reduction in group-A childhood posterior fossa ependymoma and is a powerful predictor of outcome. Acta Neuropathol 134:705–714. https://doi.org/10.1007/s00401-017-1752-4
Parker M, Mohankumar KM, Punchihewa C, Weinlich R, Dalton JD, Li Y et al (2014) C11orf95-RELA fusions drive oncogenic NF-kappaB signalling in ependymoma. Nature 506:451–455. https://doi.org/10.1038/nature13109
Piunti A, Hashizume R, Morgan MA, Bartom ET, Horbinski CM, Marshall SA et al (2017) Therapeutic targeting of polycomb and BET bromodomain proteins in diffuse intrinsic pontine gliomas. Nat Med 23:493–500. https://doi.org/10.1038/nm.4296
Puelles E, Acampora D, Lacroix E, Signore M, Annino A, Tuorto F et al (2003) Otx dose-dependent integrated control of antero-posterior and dorso-ventral patterning of midbrain. Nat Neurosci 6:453–460. https://doi.org/10.1038/nn1037
Punchihewa C, Lee R, Lin T, Orisme W, Dalton J, Aronica E et al (2014) Pediatric posterior fossa ependymomas consist of two molecular subgroups defined by gene expression and methylation profiling. Neuro Oncol 16:011
Raybaud C (2016) MR assessment of pediatric hydrocephalus: a road map. Childs Nerv Syst 32:19–41. https://doi.org/10.1007/s00381-015-2888-y
Raybaud C, Ramaswamy V, Taylor MD, Laughlin S (2015) Posterior fossa tumors in children: developmental anatomy and diagnostic imaging. Childs Nerv Syst 31:1661–1676. https://doi.org/10.1007/s00381-015-2834-z
Rodriguez D, Cheung MC, Housri N, Quinones-Hinojosa A, Camphausen K, Koniaris LG (2009) Outcomes of malignant CNS ependymomas: an examination of 2408 cases through the surveillance, epidemiology, and end results (SEER) database (1973–2005). J Surg Res 156:340–351. https://doi.org/10.1016/j.jss.2009.04.024
Ryall S, Guzman M, Elbabaa SK, Luu B, Mack SC, Zapotocky M et al (2017) H3 K27M mutations are extremely rare in posterior fossa group A ependymoma. Childs Nerv Syst 33:1047–1051. https://doi.org/10.1007/s00381-017-3481-3
Sgaier SK, Lao Z, Villanueva MP, Berenshteyn F, Stephen D, Turnbull RK et al (2007) Genetic subdivision of the tectum and cerebellum into functionally related regions based on differential sensitivity to engrailed proteins. Development 134:2325–2335. https://doi.org/10.1242/dev.000620
Sturm D, Bender S, Jones DT, Lichter P, Grill J, Becher O et al (2014) Paediatric and adult glioblastoma: multiform (epi)genomic culprits emerge. Nat Rev Cancer 14:92–107. https://doi.org/10.1038/nrc3655
Swanson WJ, Vacquier VD (2002) The rapid evolution of reproductive proteins. Nat Rev Genet 3:137–144. https://doi.org/10.1038/nrg733
Tihan T, Zhou T, Holmes E, Burger PC, Ozuysal S, Rushing EJ (2008) The prognostic value of histological grading of posterior fossa ependymomas in children: a Children’s Oncology Group study and a review of prognostic factors. Mod Pathol 21:165–177. https://doi.org/10.1038/modpathol.3800999
U-King-Im J, Taylor MD, Raybaud C (2010) Posterior fossa ependymomas: new radiological classification with surgical correlation. Childs Nerv Syst 26:1765–1772. https://doi.org/10.1007/s00381-010-1251-6
Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A et al (2015) Proteomics. Tissue-based map of the human proteome. Science 347:394. https://doi.org/10.1126/science.1260419
van der Maaten L, Hinton G (2008) Visualizing data using t-SNE. J Mach Learn Res 9:2579–2605
Vinchon M, Leblond P, Noudel R, Dhellemmes P (2005) Intracranial ependymomas in childhood: recurrence, reoperation, and outcome. Childs Nerv Syst 21:221–226
Wani K, Armstrong TS, Vera-Bolanos E, Raghunathan A, Ellison DW, Gilbertson R et al (2012) A prognostic gene expression signature in infratentorial ependymoma. Acta Neuropathol 123:727–738. https://doi.org/10.1007/s00401-012-0941-4
Wilkerson MD, Hayes DN (2010) ConsensusClusterPlus: a class discovery tool with confidence assessments and item tracking. Bioinformatics 26:1572–1573. https://doi.org/10.1093/bioinformatics/btq170
Witt H, Mack SC, Ryzhova M, Bender S, Sill M, Isserlin R et al (2011) Delineation of two clinically and molecularly distinct subgroups of posterior fossa ependymoma. Cancer Cell 20:143–157. https://doi.org/10.1016/j.ccr.2011.07.007
Wu G, Diaz AK, Paugh BS, Rankin SL, Ju B, Li Y et al (2014) The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat Genet 46:444–450. https://doi.org/10.1038/ng.2938
Xue B, Dunbrack RL, Williams RW, Dunker AK, Uversky VN (2010) PONDR-FIT: a meta-predictor of intrinsically disordered amino acids. Biochim Biophys Acta 1804:996–1010. https://doi.org/10.1016/j.bbapap.2010.01.011
Zhang J, Wu G, Miller CP, Tatevossian RG, Dalton JD, Tang B et al (2013) Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet 45:602–612. https://doi.org/10.1038/ng.2611
Acknowledgements
Support at St. Jude was provided by the American Lebanese Syrian Associated Charities and by NCI grants CA-96832 (to M.R.) and CA096832-13 (to D.W.E.). The work was supported by the CERN Research Fellowship (to K.W.P.), the ICGC PedBrain Tumour Project funded by the German Cancer Aid (109252) and the German Federal Ministry of Education and Research (to S.M.P.), the KIKA grant (#90) (to M.K.). Support at the University of Nottingham was provided by ‘Fighting Ependymoma’ and the ‘Connie and Albert Taylor Trust’.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pajtler, K.W., Wen, J., Sill, M. et al. Molecular heterogeneity and CXorf67 alterations in posterior fossa group A (PFA) ependymomas. Acta Neuropathol 136, 211–226 (2018). https://doi.org/10.1007/s00401-018-1877-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-018-1877-0