Abstract
Purpose
Cortical malformations (CMs) are increasingly recognized as the epileptogenic substrate in patients with medically refractory neocortical epilepsy (NE). The aim of this study was to test the hypotheses that: 1. CMs are metabolically heterogeneous. 2. The structurally normal appearing perilesional zone is characterized by similar metabolic abnormalities as the CM.
Methods
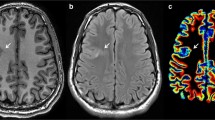
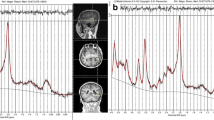
Magnetic resonance spectroscopic imaging (MRSI) in combination with tissue segmentation was performed on eight patients with NE and CMs and 19 agematched controls. In controls, NAA, Cr, Cho,NAA/Cr and NAA/Cho of all voxels of a given lobe were expressed as a function of white matter content and thresholds for pathological values determined by calculating the 95% prediction intervals. These thresholds were used to identify metabolically abnormal voxels within the CM and in the perilesional zone.
Results
30% of all voxels in the CMs were abnormal, most frequently because of decreases of NAA or increases of Cho. Abnormal voxels tended to form metabolically heterogeneous clusters interspersed in metabolically normal regions. Furthermore, 15% of all voxels in the perilesional zone were abnormal, the most frequent being decreases of NAA and Cr.
Conclusion
In CMs metabolically normal regions are interspersed with metabolically heterogeneous abnormal regions. Metabolic abnormalities in the perilesional zone share several characteristics of CMs and might therefore represent areas with microscopic malformations and/or intrinsic epileptogenicity.
Similar content being viewed by others
References
Battaglia G, Arcelli P, Granata T, et al. (1996) Neuronal migration disorders and epilepsy: a morphological analysis of three surgically treated patients. Epilepsy Res 26:49–58
Bhakoo KK, Craig TJ, Styles P (2001) Developmental and regional distribution of aspartoacylase in rat brain tissue. J Neurochem 79:211–220
Bhakoo KK, Pearce D (2000) In vitro expression of N–acetylaspartate by oligodendrocytes: Implications for proton magnetic resonance spectroscopy signal in vivo. J Neurochem 74:254–262
Boonyapisit K, Najm I, Klem G, et al. (2003) Epileptogenicity of focal malformations due to abnormal cortical development: direct electrocorticographic– histopathologic correlations. Epilepsia 44:69–76
Capizzano AA, Vermathen P, Laxer KD, et al. (2002) Multisection proton MR spectroscopy for mesial temporal lobe epilepsy. Am J Neuroradiol 23:1359–1368
Castillo M, Kwock L, Scatliff J, Gudeman S, Greenwood R (1993) Proton MR spectroscopic characteristics of a presumed giant subcortical heterotopia. Am J Neuroradiol 14:426–429
Chakraborty G, Mekala P Yahya D, Ledeen RW (2001) Intraneuronal Nacetylaspartate supplies acetyl groups for myelin lipid synthesis: evidence for myelin–associated aspartoacylase. J Neurochem 78:736–745
Chevassus–au–Louis N, Represa A (1999) The right neuron at the wrong place: biology of heterotopic neurons in cortical migration disorders with special reference to associated pathologies. Cell Mol Life Sci 55:1206–1215
Damasio H (1995) Human brain anatomy in computerized images. Oxford University Press, New York
Grant PE, Barkovich AJ, Wald LL, Dillon WP, Laxer KD, Vigneron DB (1997) High resolution surface–coil MR of cortical lesions in medically refractory epilepsy: a prospective study. Am J Neuroradiol 18:291–301
Guye M, Le Fur Y, Confort–Gouny S, et al. (2002) Metabolic and electrophysiological alterations in subtypes of temporal lobe epilepsy: a combined proton magnetic resonance spectroscopic imaging and depth electrode study. Epilepsia 43:1197–209
Hammers A, Koepp MJ, Richardson MP, et al. (2001) Central benzodiazepine receptors in malformations of cortical development. A quantitative study. Brain 124:1555–1565
Hanefeld F, Kruse B, Holzbach U, et al. (1995) Hemimegalencephaly: localized proton magnetic resonance spectroscopy in vivo. Epilepsia 36:1215–1224
Haupt CI, Schuff N, Weiner MW, Maudsley AA (1996) Removal of lipid artifacts in 1H spectroscopic imaging by data extrapolation. Magn Reson Med 35:678–687
Jacobs MA, Horská A, van Zijl PCM, Barker PB (2001) Quantitative proton MR spectroscopic imaging of normal human cerebellum and brain stem. Mag Reson Med 46:699–705
Juhász C, Chugani DC, Muszik O, et al. (2000) Electroclinical correlates of flumazenil and fluorodeoxyglucose PET abnormalities in lesional epilepsy. Neurology 55:825–834
Kuzniecky R, Hetherington H, Pan J, et al. (1997) Proton spectroscopic imaging at 4. 1 T in patients with malformations of cortical development and epilepsy. Neurology 48:1018–1024
Li LM, Cendes F, Cunha Bastos A, Andermann F, Dubeau F, Arnold DL (1998) Neuronal metabolic dysfunction in patients with cortical developmental malformations. Neurology 50:755–759
Marsh L, Lim KO, Sullivan EV, Lane B, Spielman D (1996) Proton magnetic resonance spectroscopy of a gray matter heterotopia. Neurology 47:1571–1574
Maudsley AA, Lin E, Weiner MW (1992) Spectroscopic imaging display and analysis. Magn Reson Imag 10:471–485
Mueller SG, Suhy J, Laxer KD, et al. (2002) Reduced extrahippocampal NAA in mesial temporal lobe epilepsy. Epilepsia 43:1210–1216
Najm I, Ying Z, Babb T, et al. (2004) Mechanisms of epileptogenicity in cortical dysplasias. Neurology 62 (6 Suppl 3):S9–S13
Palmini A (2000) Disorders of cortical development. Curr Opin Neurol 13:183–192
Porter BE, Brooks–Kayal A, Golden JA (2002) Disorders of cortical development and epilepsy. Arch Neurol 59:361–365
Schuff N, Ezekiel F, Gamst AC, et al. (2001) Region and tissue differences of metabolites in normally aged brain using multislice 1H magnetic resonance spectroscopic imaging. Mag Reson Med 45:899–907
Simone IL, Federico F, De Blasi R, et al. (1999) Metabolic changes in neuronal migration disorders: evaluation by combined MRI and Proton MR spectroscopy. Epilepsia 40:872–879
Soher BJ, Young K, Govindaraju V, Maudsley AA (1998) Automated spectral analysis III: Application to in vivo proton MR spectroscopy and spectroscopic imaging. Magn Reson Med 40:822–831
Tanabe JL, Amend D, Schuff N, et al. (1997) Tissue segmentation of the brain in Alzheimer disease. Am J Neuroradiol 18:115–123
Tassi L, Pasquier B, Minotti L, et al. (2001) Cortical dysplasia: electroclinical, imaging and neuropathologic study of 13 patients. Epilepsia 42:1112–1123
Urenjak J, Williams SR, Gadian DG, Noble M (1992) Specific expression of N–acetylasparate in neurons, oligodendrocyte– type–2 astrocyte progenitors and immature oligodendrocytes in vitro. J Neurochem 59:55–61
Woermann FG, McLean MA, Bartlett PA, Barker GJ, Duncan JS (2001) Qualitative short echo time proton magnetic resonance spectroscopic imaging study of malformations of cortical development causing epilepsy. Brain 124:427–436
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mueller, S.G., Laxer, K.D., Barakos, J.A. et al. Metabolic characteristics of cortical malformations causing epilepsy. J Neurol 252, 1082–1092 (2005). https://doi.org/10.1007/s00415-005-0819-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-005-0819-7