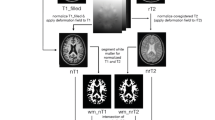
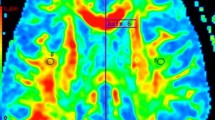
Abstract
“Dirty-appearing white matter” (DAWM) in multiple sclerosis (MS) is defined as a region(s) with ill-defined borders of intermediate signal intensity between that of normal-appearing white matter (NAWM) and that of plaque on T2-weighted and proton density imaging. To delineate the histopathology of DAWM, four formalin-fixed cerebral hemisphere slices of three MS patients with DAWM were scanned with T2- weighted and proton density sequences. The myelin water fraction (MWF) was obtained by expressing the short T2 component as a fraction of the total T2 distribution. Hemispheric sections were then stained with Luxol fast blue (LFB) for myelin phospholipids, for myelin basic protein (MBP) and 2’,3’-cyclic nucleotide 3’-phosphohydrolase (CNP) for myelin; Bielschowsky silver impregnation for axons; and for glial fibrillary acidic protein (GFAP) for astrocytes. Compared to NAWM, DAWM showed reduction in MWF, corresponding to a reduction of LFB staining. DAWM also showed reduced Bielschowsky staining. Quantitatively, the change in MWF in DAWM most consistently correlated with the change in LFB staining. The findings of this preliminary study suggest that DAWM is characterized by loss of myelin phospholipids, detected by the short T2 component, and axonal reduction.
Similar content being viewed by others
References
Allen IV, Glover G, Anderson R (1981) Abnormalities in the macroscopically normal white matter in cases of mild or spinal multiple sclerosis (MS). Acta Neuropathol Suppl (Berl) 7:176–178
Allen IV, McKeown SR (1979) A histological, histochemical and biochemical study of the macroscopically normal white matter in multiple sclerosis. J Neurol Sci 41:81–91
Allen IV, McQuaid S, Mirakhur M, Nevin G (2001) Pathological abnormalities in the normal-appearing white matter in multiple sclerosis. Neurol Sci 22:141–144
Arstila AU, Riekkinen P, Rinne UK, Laitinen L (1973) Studies on the pathogenesis of multiple sclerosis. Participation of lysosomes on demyelination in the central nervous system white matter outside plaques. Eur Neurol 9:1–20
Barnard RO, Triggs M (1974) Corpus callosum in multiple sclerosis. J Neurol Neurosurg Psychiatry 37:1259–1264
Barnett MH, Prineas JW (2004) Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann Neurol 55:458–468
Bruck W, Stadelmann C (2004) Pathology of the normal-appearing white matter in multiple sclerosis. In: Filipi M, Comi G, Rovaris M (eds) Normalappearing white and grey matter damage in multiple sclerosis. Springer- Verlag, Milan, pp 3–8
Cumings JN (1955) Lipid chemistry of the brain in demyelinating diseases. Brain 78:554–563
De Stefano N, Narayanan S, Francis SJ, Smith S, Mortilla M, Tartaglia MC, Bartolozzi ML, Guidi L, Federico A, Arnold DL (2002) Diffuse axonal and tissue injury in patients with multiple sclerosis with low cerebral lesion load and no disability. Arch Neurol 59:1565–1571
Evangelou N, Esiri MM, Smith S, Palace J, Matthews PM (2000) Quantitative pathological evidence for axonal loss in normal appearing white matter in multiple sclerosis. Ann Neurol 47:391–395
Evangelou N, Konz D, Esiri MM, Smith S, Palace J, Matthews PM (2000) Regional axonal loss in the corpus callosum correlates with cerebral white matter lesion volume and distribution in multiple sclerosis. Brain 123(Pt 9):1845–1849
Fazekas F, Barkhof F, Filippi M, Grossman RI, Li DK, McDonald WI, McFarland HF, Paty DW, Simon JH, Wolinsky JS, Miller DH (1999) The contribution of magnetic resonance imaging to the diagnosis of multiple sclerosis. Neurology 53:448–456
Fernando KT, McLean MA, Chard DT, MacManus DG, Dalton CM, Miszkiel KA, Gordon RM, Plant GT, Thompson AJ, Miller DH (2004) Elevated white matter myo-inositol in clinically isolated syndromes suggestive of multiple sclerosis. Brain 127:1361–1369
Filippi M, Campi A, Dousset V, Baratti C, Martinelli V, Canal N, Scotti G, Comi G (1995) A magnetization transfer imaging study of normal-appearing white matter in multiple sclerosis. Neurology 45:478–482
Filippi M, Comi G, Rovaris M (2004) Normal-appearing white matter and grey matter damage in multiple sclerosis. Springer-Verlag, Milan
Fog T (1965) The topography of plaques in multiple sclerosis with special reference to cerebral plaques. Acta Neurol Scand Suppl 15:1–161
Ge Y, Grossman RI, Babb JS, He J, Mannon LJ (2003) Dirty-appearing white matter in multiple sclerosis: volumetric MR imaging and magnetization transfer ratio histogram analysis. AJNR Am J Neuroradiol 24:1935–1940
Gendelman HE, Pezeshkpour GH, Pressman NJ, Wolinsky JS, Quarles RH, Dobersen MJ, Trapp BD, Kitt CA, Aksamit A, Johnson RT (1985) A quantitation of myelin-associated glycoprotein and myelin basic protein loss in different demyelinating diseases. Ann Neurol 18:324–328
Gerstl B, Kahnke MJ, Smith JK, Tavaststjerna MG, Hayman RB (1961) Brain lipids in multiple sclerosis and other diseases. Brain 84:310–319
Gobin SJ, Montagne L, Van Zutphen M, Van Der Valk P, Van Den Elsen PJ, De Groot CJ (2001) Upregulation of transcription factors controlling MHC expression in multiple sclerosis lesions. Glia 36:68–77
Guo AC, Jewells VL, Provenzale JM (2001) Analysis of normal-appearing white matter in multiple sclerosis: comparison of diffusion tensor MR imaging and magnetization transfer imaging. AJNR Am J Neuroradiol 22:1893–1900
Husted CA, Goodin DS, Hugg JW, Maudsley AA, Tsuruda JS, de Bie SH, Fein G, Matson GB, Weiner MW (1994) Biochemical alterations in multiple sclerosis lesions and normal-appearing white matter detected by in vivo 31P and 1H spectroscopic imaging. Ann Neurol 36:157–165
Inglese M, Li BS, Rusinek H, Babb JS, Grossman RI, Gonen O (2003) Diffusely elevated cerebral choline and creatine in relapsing-remitting multiple sclerosis. Magn Reson Med 50:190–195
Itoyama Y, Sternberger NH, Webster HD, Quarles RH, Cohen SR, Richardson EP Jr (1980) Immunocytochemical observations on the distribution of myelin-associated glycoprotein and myelin basic protein in multiple sclerosis lesions. Ann Neurol 7:167–177
Johnson D, Sato S, Quarles RH, Inuzuka T, Brady RO, Tourtellotte WW (1986) Quantitation of the myelinassociated glycoprotein in human nervous tissue from controls and multiple sclerosis patients. J Neurochem 46:1086–1093
Kirk J, Plumb J, Mirakhur M, McQuaid S (2003) Tight junctional abnormality in multiple sclerosis white matter affects all calibres of vessel and is associated with blood-brain barrier leakage and active demyelination. J Pathol 201:319–327
Kluver H, Barrera E (1953) A method for the combined staining of cells and fibers in the nervous system. J Neuropathol Exp Neurol 12:400–403
Kutzelnigg A, Lucchinetti CF, Stadelmann C, Bruck W, Rauschka H, Bergmann M, Schmidbauer M, Parisi JE, Lassmann H (2005) Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 128:2705–2712
Laule C, Leung E, Li DKB, Traboulsee AL, MacKay AL, Moore GW (2004) Myelin water imaging in multiple sclerosis: quantitative correlations with histopathology. In: Proceedings of the 12th Annual Meeting of International Society for Magnetic Resonance in Medicine (ISMRM). Kyoto, Japan, p 461
Laule C, Leung E, Li DK, Traboulsee AL, Paty DW, MacKay AL, Moore GR (2006) Myelin water imaging in multiple sclerosis: quantitative correlations with histopathology. Mult Scler 12:747–753
Laule C, Vavasour IM, Moore GR, Oger J, Li DK, Paty DW, MacKay AL (2004) Water content and myelin water fraction in multiple sclerosis. A T2 relaxation study. J Neurol 251:284–293
Lindberg RL, De Groot CJ, Montagne L, Freitag P, van der Valk P, Kappos L, Leppert D (2001) The expression profile of matrix metalloproteinases (MMPs) and their inhibitors (TIMPs) in lesions and normal appearing white matter of multiple sclerosis. Brain 124:1743–1753
Lycette RM, Danforth WF, Koppel JL, Olwin JH (1970) The binding of luxol fast blue ARN by various biological lipids. Stain Technol 45:155–160
MacKay A, Whittall K, Adler J, Li D, Paty D, Graeb D (1994) In vivo visualization of myelin water in brain by magnetic resonance. Magn Reson Med 31:673–677
Miller DH, Grossman RI, Reingold SC, McFarland HF (1998) The role of magnetic resonance techniques in understanding and managing multiple sclerosis. Brain 121(Pt 1):3–24
Miller DH, Thompson AJ, Filippi M (2003) Magnetic resonance studies of abnormalities in the normal appearing white matter and grey matter in multiple sclerosis. J Neurol 250:1407–1419
Miropolosky V VT, Zhao G, Zhao Y, Traboulsee A, Li DKB (2006) Dirty-appearing white matter in multiple sclerosis: relationship to T2 disease burden increase and brain volume decrease with 8-year long-term followup (abstract). Mult Scler 12(Suppl 1):S174
Moore GR, Leung E, MacKay AL, Vavasour IM, Whittall KP, Cover KS, Li DK, Hashimoto SA, Oger J, Sprinkle TJ, Paty DW (2000) A pathology-MRI study of the short-T2 component in formalin-fixed multiple sclerosis brain. Neurology 55:1506–1510
Moore GRW (2003) MRI-clinical correlations: more than inflammation alone – what can MRI contribute to improve the understanding of pathological processes in MS? Journal of the Neurological Sciences 206:175–179
Narayana PA, Doyle TJ, Lai D, Wolinsky JS (1998) Serial proton magnetic resonance spectroscopic imaging, contrastenhanced magnetic resonance imaging, and quantitative lesion volumetry in multiple sclerosis. Ann Neurol 43:56–71
Narayana PA, Wolinsky JS, Rao SB, He R, Mehta M, PROMISE Trial MRSI Group (2004) Multicentre proton magnetic resonance spectroscopy imaging of primary progressive multiple sclerosis. Mult Scler 10(Suppl 1):S73–78
Norton WT, Cammer W (1984) Chemical pathology of diseases involving myelin. In: Morell P (ed) Myelin 2nd ed. Plenum, New York, pp 369–403
Plumb J, McQuaid S, Mirakhur M, Kirk J (2002) Abnormal endothelial tight junctions in active lesions and normalappearing white matter in multiple sclerosis. Brain Pathol 12:154–169
Poon CS, Henkelman RM (1992) Practical T2 quantitation for clinical applications. J Magn Reson Imaging 2:541–553
Prineas JW, Kwon EE, Sternberger NH, Lennon VA (1984) The distribution of myelin-associated glycoprotein and myelin basic protein in actively demyelinating multiple sclerosis lesions. J Neuroimmunol 6:251–264
Riekkinen PJ, Rinne UK, Arstila AU (1972) Neurochemical and morphological studies on demyelination in multiple sclerosis with special reference to etiological aspects. Z Neurol 203:91–104
Rinne UK, Riekkinen PJ, Arstila AU (1972) Biochemical and electron microscopic alterations in the white matter outside demyelinated plaques in multiple sclerosis. In: Lubowitz U (ed) Progress in Multiple Sclerosis. Academic Press, New York, pp 76–98
Ropele S, Strasser-Fuchs S, Augustin M, Stollberger R, Enzinger C, Hartung HP, Fazekas F (2000) A comparison of magnetization transfer ratio, magnetization transfer rate, and the native relaxation time of water protons related to relapsing-remitting multiple sclerosis. AJNR Am J Neuroradiol 21:1885–1891
Salthouse TN (1962) Luxol fast blue ARN: a new solvent azo dye with improved staining qualities for myelin and phospholipids. Stain Technol 37:313–316
Segarra JM (1970) Histological and histochemical staining methods: A selection. In: Teduchi CG (ed) Neuropathology methods and diagnosis. Little, Brown and Company, Boston, pp 233–269
Suzuki K, Kamoshita S, Eto Y, Tourtellotte WW, Gonatas JO (1973) Myelin in multiple sclerosis. Composition of myelin from normal-appearing white matter. Arch Neurol 28:293–297
Traboulsee A, Dehmeshki J, Peters KR, Griffin CM, Brex PA, Silver N, Ciccarrelli O, Chard DT, Barker GJ, Thompson AJ, Miller DH (2003) Disability in multiple sclerosis is related to normal appearing brain tissue MTR histogram abnormalities. Mult Scler 9:566–573
van Walderveen MA, van Schijndel RA, Pouwels PJ, Polman CH, Barkhof F (2003) Multislice T1 relaxation time measurements in the brain using IREPI: reproducibility, normal values, and histogram analysis in patients with multiple sclerosis. J Magn Reson Imaging 18:656–664
Vos CM, Geurts JJ, Montagne L, van Haastert ES, Bo L, van der Valk P, Barkhof F, de Vries HE (2005) Blood-brain barrier alterations in both focal and diffuse abnormalities on postmortem MRI in multiple sclerosis. Neurobiol Dis 20:953–960
Werring DJ, Clark CA, Droogan AG, Barker GJ, Miller DH, Thompson AJ (2001) Water diffusion is elevated in widespread regions of normal-appearing white matter in multiple sclerosis and correlates with diffusion in focal lesions. Mult Scler 7:83–89
Whittall KP, MacKay AL, Li DK, Vavasour IM, Jones CK, Paty DW (2002) Normal-appearing white matter in multiple sclerosis has heterogeneous, diffusely prolonged T(2). Magn Reson Med 47:403–408
Zhao G, Li DKB, Cheng Y, Paty DW, UBC MS Research Group. PRISMS Study Group (2003) Possible prognostic significance of dirty-appearing white matter on MRI in multiple sclerosis (abstract). Mult Scler 9(Suppl 1):S61
Zhao GJ, Li DKB, Cheng Y, Wang XY, Paty DW (2000) MRI dirty-appearing white matter in MS. (Abstract). Neurology 54(Suppl 3):A121
Author information
Authors and Affiliations
Corresponding author
Additional information
† Deceased
Rights and permissions
About this article
Cite this article
Moore, G.R.W., Laule, C., MacKay, A. et al. Dirty-appearing white matter in multiple sclerosis. J Neurol 255, 1802–1811 (2008). https://doi.org/10.1007/s00415-008-0002-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-008-0002-z