Abstract
Although there is substantial brain grey matter pathology in secondary progressive multiple sclerosis (MS), there has been limited investigation of its contribution to disability.
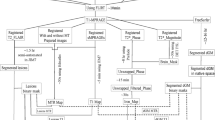
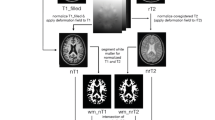
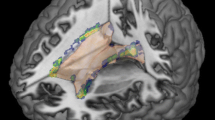
This study investigated the correlation of magnetization transfer ratio (MTR) measures taken from brain grey matter, normal appearing white matter (NAWM) and lesions with neurological deficit and disability in 113 people with secondary progressive MS. In order to adjust for the potential effects of focal white matter lesions and global brain atrophy, T2 lesion volume and normalized brain volume (NBV) were also calculated for each subject. Clinical measures included the expanded disability status scale (EDSS) and the multiple sclerosis functional composite (MSFC) scores. Linear regression analysis was used to assess the age- and gender-adjusted correlation of MTR histogram mean, peak height and peak location with the MSFC and individual component measures. Logistic regression analysis was used to determine whether imaging measures could be used to predict if subjects were in the higher disability group (EDSS ≥ 6.5).
Significant correlations were detected between MSFC composite and mean MTR in (i) normal appearing white matter (NAWM; r = 0.327, p < 0.0001), (ii) grey matter (r = 0.460, p < 0.0001) and (iii) lesions (r = 0.394, p < 0.0001). Although NBV and T2 lesion volume correlated significantly with MSFC, grey matter histogram mean emerged as the best predictor of MSFC score. None of the MRI measures significantly predicted higher EDSS.
These results suggest that brain grey matter pathology plays an important role in determining neurological impairment. The apparent paucity of correlation between MRI measures and EDSS is likely to represent the relative insensitivity of the latter measure in this study group.
Similar content being viewed by others
References
Agosta F, Rovaris M, Pagani E, Sormani MP, Comi G, Filippi M (2006) Magnetization transfer MRI metrics predict the accumulation of disability 8 years later in patients with multiple sclerosis. Brain 129:2620–2627
Ashburner J, Friston K (1997) Multimodal image coregistration and partitioning – a unified framework. NeuroImage 6:209–217
Barker GJ, Tofts PS, Gass A (1996) An interleaved sequence for accurate and reproducible clinical measurement of magnetization transfer ratio. Magn Res Im 14:403–411
Barkhof F, Bruck W, De Groot CJA, Bergers E, Hushof S, Geurts J, Polman CH, van der Valk P (2003) Remyelinated lesions in multiple sclerosis. Arch Neurol 60:1073–1081
Beniek M, Altmann DR, Davies GR, Ingle GT, Rashid W, Sastre-Garriga A, Thompson AJ, Miller DH (2006) Cord atrophy separates early primary progressive and relapsing remitting multiple sclerosis. JNNP 77:1036–1039
Bjartmar C, Trapp BD (2002) Axonal and neuronal degeneration in multiple sclerosis: mechanisms and functional consequences. Curr Opin Neurol 51:311–318
Bo L, Vedeler CA, Nyland H, Trapp BD, Mork SJ (2003) Intracortical multiple sclerosis lesions are not associated with increased lymphocyte infiltration. Mult Scler 9:323–331
Brownell B, Hughes J (1962) The distribution of plaques in cerebrum in multiple sclerosis. JNNP 25:315–320
Cercignani M, Bozzali M, Iannucci G, Comi G, Filippi M (2001) Magnetization transfer ratio and mean diffusivity of normal appearing white matter and grey matter from parents with multiple sclerosis. JNNP 70:311–317
Chard DT, Griffin CM, Parker GJM, Kapoor R, Thompson AJ, Miller DH (2002) Brain atrophy in clinically early relapsing-remitting multiple sclerosis. Brain 125:327–337
Cifelli A, Arridge M, Jezzard P, Esiris MM, Palace J, Matthew PM (2002) Thalamic neurodegeneration in multiple sclerosis. Ann Neurol 52:650–653
Cutter GR, Baier ML, Rudick RA, Cookfair DL, Fischer JS, Petkau J, Syndulko K, Weinshenker AE, Antel JP, Confavreaux C, Ellison GW, Lublin F, Miller AE, Rao SM, Reingold S, Thompson A, Willoughby E (1999) Development of a multiple sclerosis functional composite as a clinical trail outcome measure. Brain 122:871–882
Davies GR, Ramio-Torrenta L, Hadjiprocopis A, Chard DT, Griffin CMB, Rashid W, Barker GJ, Kapoor R, Thompson AJ, Miller DH (2004) Evidence for grey matter MTR abnormality in minimally disabled patients with early relapsing-remitting multiple sclerosis. JNNP 75:998–1002
Davies GR, Altmann DR, Hadjiprocopis A, Rashid W, Chard DT, Griffin CM, Tofts PS, Barker GJ, Kapoor R, Thompson AJ, Miller DH (2005) Increasing normal appearing grey and white matter magnetisation transfer ratio abnormality id early relapsing-remitting multiple sclerosis. J Neurol 252:1037–1044
Dehmeshki J, Ruto AC, Arridge S, Silver NC, Miller DH, Tofts PS (2001) Analysis of MTR histograms in multiple sclerosis using principle components and multiple discriminant analysis. MRM 46:600–609
Dehmeshki J, Chard DT, Leary SM, Watt HC, Silver NC, Tofts PS, Thompson AJ, Miller DH (2003) The Normal appearing grey matter in primary progressive multiple sclerosis. A Magnetisation transfer imaging study. J Neurol 250:67–74
Evangelou N, Esiri MM, Smith S, Palace J, Matthews PM (2000) Quantitative pathological evidence for axonal loss in normal appearing white matter in multiple sclerosis. Ann Neurol 47(3):391–395
Filippi M, Campi A, Dousset V, Baratti C, Martinelli V, Canal N, Scotti G, Comi G (1995) A magnetization transfer imaging study of normal appearing white matter in multiple sclerosis. Neurology 45:478–482
Filippi M, Rocca MA, Pagani E (2004) European study on intravenous immunoglobulin in multiple sclerosis. Results of Magnetization Transfer Magnetic Resonance Imaging Analysis. Arch Neurol 61:1409–1412
Fox NC, Jenkins R, Leary SM, Stevenson VL, Losseff NA, Crum WR, Harvey RJ, Rossor MN, Miller DH, Thompson AJ (2000) Progressive cerebral atrophy in MS. A serial study using registered volumetric MRI. Neurology 54:807–812
Furby J, HaytonT, Anderson V, Altmann D, Brenner R, Chataway J, Hughes RAC, Smith KJ, Miller DH, Kapoor (2008) Magnetice resonance imaging measures of brain and spinal cord atrophy correlate with clinical impairment in secondary progressive multiple sclerosis. Mult Scler 14:1068–1075
Gass A, Barker GJ, Kidd D, Thorpe JW, MacManus D, Brennan A, Tofts PS, Thompson AJ, McDonald WI, Miller DH (1994) Correlation of magnetization transfer ratio with clinical disability in multiple sclerosis. Ann Neurol 36:62–67
Ge Y, Grossman RI, Udupa JK, Babb JS, Manon LJ, McGowan JC (2002) Magnetization transfer ratio histogram analysis of normal-appearing gray matter and normal-appearing white matter in multiple sclerosis. J CAT 26:61–68
Geurts JJG, Bo L, Puowels PJW, Castelijns JA, Polman CH, Barkhof F (2005) Cortical lesions in multiple sclerosis: Combined postmortem MR imaging and histopathology. Am J Neuroradiol 26:572–577
Griffin CM, Chard DT, Parker GJM, Barker GJ, Thompson AJ, Miller DH (2002) The relationship between lesion and normal appearing brain tissue abnormalities in early relapsing remitting multiple sclerosis. J Neurol 249:193–199
Inglese M, van Easberghe JHTM, Rovaris M, Beckmann K, Barkhof F, Hahn D, Kappos L, Miller DH, Polman C, Pozilli C, Thompson AJ, Yousry TA, Wagner K, Comi C, Filippi M (2003) The effect of interferon β-1b on quantities derived from MT MRI in secondary progressive MS. Neurology 60:853–860
Kalkers NF, Bergers E, Castelijns JA, van Walderween MAA, Bot JCJ, Ader HJ, Polman CH, Barkhof F (2001) Optimising the association between disability and biological markers in MS. Neurology 57:1253–1258
Kalkers NF, Hintzen RQ, van Waesberghe JHTM, Lazeron RHC, van Schijndel RA, Ader HJ, Polman CH, Barkhof F (2001) Magnetization transfer histogram parameters reflect all dimensions of MS pathology including atrophy. J Neurol Sci 184:155–162
Kidd D, Barkhof F, McConnell R, Algra PR, Allen IV, Revesz T (1999) Cortical lesions in multiple sclerosis. Brain 122:17–26
Kutzelnigg A, Luchinetti CF, Stadelman C, Bruck W, Rauschka H, Bermann M, Schmidbauer M, Parisi JE, Lassman H (2005) Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 128:2705–2712
Li DKB, Held U, Petkau J, Daumer M, Barkhof F, Fazekas F, Frank JA, Kappos L, Miller DH, Simon JH, Wolinsky JS, Filippi M, for the Sylvia Lawry Centre for MS Research (2006) MRI T2 lesion burden in multiple sclerosis: a plateauing relationship with clinical disability. Neurology 66:1384–1389
Lovas G, Szilagyi N, Majtenya K, Palkovits M, Komoly S (2000) Axonal changes in chronic demyelinated cervical spinal cord plaques. Brain 123:308–317
Lumsden CE (1970) The neuropathology of multiple sclerosis. In: Vinken PI, Bruyn GW (eds) Handbook of clinical neurology. Elsevier, New York, pp 217–309
Miller DH, Barkhof F, Frank JA, Parker GJM, Thompson AJ (2002) Measurement of atrophy in multiple sclerosis: pathological basis, methodological aspects and clinical relevance. Brain 125:1676–1695
Oppenheimer DR (1978) The cervical cord in multiple sclerosis. Neuropath Appl Neurobiol 4:151–162
Peterson JW, Bo L, Mork S, Chang A, Trapp BD (2001) Transected neurites, apoptotic neurons and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol 50:389–400
Quantarelli M, Ciarmello A, Brescia Morra V, Orefice G, Larobina M, Lanzillo R, Schiavone V, Salvatore E, Alfano B, Brunetti A (2003) Brain tissue volume changes in relapsing remitting multiple sclerosis: correlation with lesion load. NeuroImage 18:360–366
Ramio-Torrenta L, Sastre Garriga J, Ingle GT, Davies GR, Ameen V, Miller DH, Thompson AJ (2006) Abnormalities in normal appearing tissues in early primary progressive multiple sclerosis and their relation to disability: a tissue specific magnetisation transfer study. JNNP 77:40–45
Rovaris M, Bozzali M, Santuccio G, Ghezzi A, Caputo D, Montanari E, Bertolotto A, Bergamaschi R, capra R, Mancardi G, Martinelli V, Comi G, Filippi M (2001) In vivo assessment of the brain and cervical cord pathology in patients with primary progressive multiple sclerosis. Brain 124:2540–2549
Sanfilipo MP, Benedict RH, Sharma J, Weinstock-Guttmen B, Bakshi R (2005) The relationship between whole brain volume and disability in multiple sclerosis: a comparison of normalized grey vs. White matter with misclassification. Neuroimage 26:1068–1077
Santos AC, Narayanan S, de Stefano N, Tartaglia MC, Francis SJ, Arnoutelis R, Caramanos Z, Antel JP, Pike GB, Arnold DL (2002) Magnetization transfer can predict clinical evolution in patients with multiple sclerosis. J Neurol 249:662–668
Schmierer K, Scaravelli F, Altmann DR, Barker GJ, Miller DH (2004) Magnetization transfer ratio and myelin in postmortem multiple sclerosis brain. Ann Neurol 56:407–415
Smith SM, Zhang Y, Jenkinson M, Chen J, Matthews PM, Federico A, De Stefano N (2002) Accurate, robust and automated longitudinal and cross-sectional brain change analysis. NeuroImage 17:479–489
Tortorella C, Viti B, Bozzali M, Sormani P, Rizzo G, Gilardi MF, Comi G, Filippi M (2000) A magnetization transfer histogram study of normal appearing brain tissue in MS. Neurology 54:186–193
Tozer D, Ramani A, Barker GJ, Davies G, Miller DH, Tofts PS (2003) Quantitative magnetisation transfer mapping of bound protons in multiple sclerosis. Magm Reson Med 50:83–91
Traboulsee A, Dehmeshki J, Peters KR, Griffin CM, Brex PA, Silver N, Ciccarelli O (2003) Chard DT, Barker GJ, Thompson AJ, Miller DH. Disability in multiple sclerosis is related to normal appearing brain tissue MTR histogram analysis. Mult Scler 8:566–573
van Waesberghe JHTM, Kamphorst W, De Groot CJA, van Walderveen MAA, Castelijns JA, Ravid R Lycklama a Nijeholt GJ, van der Valk P, Polman CH, Thompson AJ, Barkhof F (1999) Axonal loss in multiple sclerosis lesions: Magnetic resonance imaging insights into substrates of disability. Ann Neurol 46:747–754
Vrenken H, Pouwels PJW, Ropele S, Knol DL, Geurts JJG, Polman CH, Barkhof F, Castelijns JA (2007) Magnetization transfer ratio measurement in multiple sclerosis normal appearing brain tissue: limited differences with controls but relationaships with clinical and MR measures of disease. Mult Scler 13:708–716
Wolff SD, Balaban RS (1989) Magnetization transfer contrast (MTC) and tissue water proton relaxation in vivo. Magn Reson Med 10:135–144
Young IR, Hall AS, Pallis CA, Legg NI, Bydder GM, Steiner RE (1981) Nuclear magnetic resonance imaging of the brain in multiple sclerosis. Lancet ii:1063–1066
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hayton, T., Furby, J., Smith, K.J. et al. Grey matter magnetization transfer ratio independently correlates with neurological deficit in secondary progressive multiple sclerosis. J Neurol 256, 427–435 (2009). https://doi.org/10.1007/s00415-009-0110-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-009-0110-4