Abstract
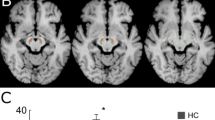
Prior work has shown that adiabatic T1ρ and T2ρ relaxation time constants may have sensitivity to cellular changes and the presence of iron, respectively, in Parkinson’s disease (PD). Further understanding of these magnetic resonance imaging (MRI) methods and how they relate to measures of disease severity and progression in PD is needed. Using T1ρ and T2ρ on a 4T MRI scanner, we assessed the substantia nigra (SN) of nine non-demented moderately affected PD and ten gender- and age-matched control participants. When compared to controls, the SN of PD subjects had increased T1ρ and reduced T2ρ. We also found a significant correlation between asymmetric motor features and asymmetry based on T1ρ. This study provides additional validation of T1ρ and T2ρ as a means to separate PD from control subjects, and T1ρ may be a useful marker of asymmetry in PD.



Similar content being viewed by others
References
Zecca L, Stroppolo A, Gatti A, Tampellini D, Toscani M, Gallorini M et al (2004) The role of iron and copper molecules in the neuronal vulnerability of locus ceruleus and substantia nigra during aging. Proc Natl Acad Sci USA 101(26):9483–9848
Faucheux BA, Martin ME, Beaumont C, Hauw JJ, Agid Y, Hirsch EC (2003) Neuromelanin associated redox-active iron is increased in the substantia nigra of patients with Parkinson’s disease. J Neurochem 86(5):1142–1148
Kaur D, Lee D, Ragapolan S, Andersen JK (2009) Glutathione depletion in immortalized midbrain-derived dopaminergeric neurons results in increases in the labile iron pool: implications for Parkinson’s disease. Free Radic Biol Med 46(5):593–598
Zecca L, Casella L, Albertini A, Bellei C, Zucca FA, Engelen M et al (2008) Neuromelanin can protect against iron-mediated oxidative damage in system modeling iron overload of brain aging and Parkinson’s disease. J Neurochem 106(4):1866–1875
Rhodes SL, Ritz B (2008) Genetics of iron regulation and the possible role of iron in Parkinson’s disease. Neurobiol Dis 32(2):183–195
Salazar J, Mena N, Hunot S, Prigent A, Alvarez-Fischer D, Arrendondo M et al (2008) Divalent metal transporter 1 (DMT1) contributes to neurodegeneration in animal models of Parkinson’s disease. Proc Natl Acad Sci USA 105(47):18578–18583
Hutchinson M, Raff U (2008) Detection of Parkinson’s disease by MRI: Spin–lattice distribution imaging. Mov Disord 23(14):1991–1997
Michaeli S, Oz G, Sorce DJ, Garwood M, Ugurbil K, Majestic S et al (2007) Assessment of brain iron and neuronal integrity in patients with Parkinson’s disease using novel MRI contrasts. Mov Disord 22(3):334–340
Gorell JM, Ordidge RJ, Brown GG, Deniau JC, Buderer NM, Helpern JA (1995) Increased iron-related MRI contrast in the substantia nigra in Parkinson’s disease. Neurology 45(6):1138–1143
Martin WR, Wieler M, Gee M (2008) Midbrain iron content in early Parkinson disease: a potential biomarker of disease status. Neurology 70(16):1411–1417
Wallis LI, Paley MN, Graham JM, Grunewald RA, Wignall EL, Joy HM et al (2008) MRI assessment of basal ganglia iron deposition in Parkinson’s disease. J Magn Reson Imaging 28(5):1061–1067
Gelb D, Oliver E, Gilman S (1999) Diagnostic criteria for Parkinson disease. Arch Neurol 56(1):33–39
Fahn S, Elton RL, members of the UPDRS Development Committee (1987) Unified Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M (eds) Recent developments in Parkinson’s disease. Macmillan Healthcare Information, Florham Park, pp 153–163
Folstein M, Folstein S, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12(3):189–198
Michaeli S, Grohn H, Grohn O, Sorce DJ, Kauppinen R, Springer CS Jr et al (2005) Exchange-influenced T2rho contrast in the human brain images measured with adiabatic radio frequency pulses. Magn Reson Med 53(4):823–829
Haase Snapshot A, MRI FLASH (1990) Applications to T1, T2, and chemical-shift imaging. Magn Reson Med 13(1):77–89
Silver MS, Joseph RI, Chen CN, Sank VJ, Hoult DI (1984) Selective population inversion in NMR. Nature 310(5979):681–683
Damier P, Hirsch E, Agid Y, Graybiel A (1999) The substantia nigra of the human brain II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain 122:1437–1448
Foster ER, Black KJ, Antenor-Dorsey JA, Perlmutter JS, Hershey T (2008) Motor asymmetry and substantia nigra volume are related to spatial delayed response performance in Parkinson’s disease. Brain Cogn 67(1):1–10
Haacke EM, Cheng NYC, House MJ, Liu Q, Neelavalli J, Ogg RJ et al (2005) Imaging iron stores in the brain using magnetic resonance imaging. Magn Reson Imaging 23(1):25
Sofic E, Riederer P, Heinsen H, Beckmann H, Reynolds GP, Hebenstreit G et al (1988) Increased iron (III) and total iron content in postmortem substantia nigra of parkinsonian brain. J Neural Transm 74(3):199–205
Grohn OH, Lukkarinen JA, Silvennoinen MJ, Pitkanen A, van Zijl PC, Kauppinen RA (1999) Quantitative magnetic resonance imaging assessment of cerebral ischemia in rat using on-resonance T(1) in the rotating frame. Magn Reson Med 42(2):268–276
Michaeli S, Burns TC, Kudishevich E, Hanson T, Sorce DJ, Garwood M et al (2009) Detection of neuronal loss using T1rho MRI assessment of 1H2O spin dynamics in the aphakia mouse. J Neurosci Methods 177(1):160–167
Borthakur A, Sochor M, Davatzikos C, Trojanowski JQ, Clark CM (2008) T1rho MRI of Alzheimer’s disease. Neuroimage 41(4):1199–1205
Michaeli S, Burns TC, Kudishevich E, Harel N, Hanson T, Sorce DJ et al (2009) Detection of neuronal loss using T1rho MRI assesment of 1H2O spin dynamics in the aphakia mouse. J Neurosci Methods 177(1):160–167
Berg D, Merz B, Reiners K, Naumann M, Becker G (2005) Five-year follow-up study of hyperechogenicity of the substantia nigra in Parkinson’s disease. Mov Disord 20(3):383–385
Leenders KL, Salmon EP, Tyrrell P, Perani D, Brooks DJ, Sager H et al (1990) The nigrostriatal dopaminergic system assessed in vivo by positron emission tomography in healthy volunteer subjects and patients with Parkinson’s disease. Arch Neurol 47(12):1290–1298
Pirker W, Holler I, Gerschlager W, Asenbaum S, Zettinig G, Brucke T (2003) Measuring the rate of progression of Parkinson’s disease over a 5-year periods with beta-CIT SPECT. Mov Disord 18(11):1266–1272
Benamer HT, Patterson J, Wyper DJ, Hadley DM, Macphee GJ, Grosset DG (2000) Correlation of Parkinson’s disease severity and duration with 123I-FP-CIT SPECT striatal uptake. Mov Disord 15(4):692–698
Acknowledgments
This study was supported by the University of Minnesota Academic Health Center, the University of Minnesota Undergraduate Research Opportunities Program, the University of Minnesota Medical Foundation, the National Institute of Dental & Craniofacial Research (T32DE007288), the National Institutes of Health (R01NS061866), and the Department of Veterans’ Affairs. We would like to acknowledge Heidi Vander Velden for her work as study coordinator and Dr. Michael A. Kuskowski (Minneapolis VA Medical Center, GRECC) for his assistance with the statistical analyses. T. Liimatainen was funded by the Instrumentarium Science Foundation, Orion Corporation Research Foundation, Finnish Cultural Foundation Northern Savo, and NIH grants P30 NS057091, P41 RR008079, R01NS061866 and R21NS059813.
Conflict of interest statement
The authors report no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nestrasil, I., Michaeli, S., Liimatainen, T. et al. T1ρ and T2ρ MRI in the evaluation of Parkinson’s disease. J Neurol 257, 964–968 (2010). https://doi.org/10.1007/s00415-009-5446-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-009-5446-2