Abstract
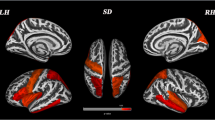
The pathology of Parkinson’s disease (PD) is not confined to the nigrostriatal dopaminergic pathway, but also involves widespread cerebral cortical areas. Such non-nigrostriatal lesions may contribute to disabling dopa-resistant parkinsonian motor deficits. We performed cortical thickness analysis to identify cerebral cortical brain areas in which thickness correlates with the severity of parkinsonian motor deficits. We performed T1-weighted brain magnetic resonance imaging studies in 142 PD patients. Motor scores on the Unified Parkinson’s Disease Rating Scale (UPDRS) were measured, and subscores were calculated for bradykinesia, rigidity, tremor, and axial motor deficits. Using FreeSurfer software, we studied cortical areas in which thickness correlates with disease duration or the severity of parkinsonian motor deficits. The cortical thickness of the parieto-temporal association cortex, including the inferior parietal and posterior parietal cortices, showed a negative correlation with disease duration, total UPDRS motor score, and UPDRS subscores for bradykinesia and axial motor deficits. We found no cortical areas in which thickness correlated with subscores for tremor and rigidity. In addition to nigrostriatal dopaminergic deficit, progressive thinning of the parieto-temporal sensory association cortices related to disease duration seems to be related in part to the exacerbation of bradykinesia and the axial motor symptoms of PD.


Similar content being viewed by others
References
Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211
Abe Y, Kachi T, Kato T, Arahata Y, Yamada T, Washimi Y, Iwai K, Ito K, Yanagisawa N, Sobue G (2003) Occipital hypoperfusion in Parkinson’s disease without dementia: correlation to impaired cortical visual processing. J Neurol Neurosurg Psychiatry 74:419–422
Bohnen NI, Minoshima S, Giordani B, Frey KA, Kuhl DE (1999) Motor correlates of occipital glucose hypometabolism in Parkinson’s disease without dementia. Neurology 52:541–546
Hu MT, Taylor-Robinson SD, Chaudhuri KR, Bell JD, Labbe C, Cunningham VJ, Koepp MJ, Hammers A, Morris RG, Turjanski N, Brooks DJ (2000) Cortical dysfunction in non-demented Parkinson’s disease patients: a combined 31P-MRS and 18FDG-PET study. Brain 123:340–352
Lyoo CH, Ryu YH, Lee MS (2010) Topographical distribution of cerebral cortical thinning in patients with mild Parkinson’s disease without dementia. Mov Disord 25:496–499
Mink JW (1996) The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol 50:381–425
Greffard S, Verny M, Bonnet AM, Beinis JY, Gallinari C, Meaume S, Piette F, Hauw JJ, Duyckaerts C (2006) Motor score of the unified Parkinson disease rating scale as a good predictor of Lewy body-associated neuronal loss in the substantia nigra. Arch Neurol 63:584–588
Apaydin H, Ahlskog JE, Parisi JE, Boeve BF, Dickson DW (2002) Parkinson disease neuropathology: later-developing dementia and loss of the levodopa response. Arch Neurol 59:102–112
Braak H, Rub U, Jansen Steur EN, Del Tredici K, de Vos RA (2005) Cognitive status correlates with neuropathologic stage in Parkinson disease. Neurology 64:1404–1410
Agid Y, Graybiel AM, Ruberg M, Hirsch E, Blin J, Dubois B, Javoy-Agid F (1990) The efficacy of levodopa treatment declines in the course of Parkinson’s disease: do nondopaminergic lesions play a role? Adv Neurol 53:83–100
Bonnet AM, Loria Y, Saint-Hilaire MH, Lhermitte F, Agid Y (1987) Does long-term aggravation of Parkinson’s disease result from nondopaminergic lesions? Neurology 37:1539–1542
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184
Vingerhoets FJ, Villemure JG, Temperli P, Pollo C, Pralong E, Ghika J (2002) Subthalamic DBS replaces levodopa in Parkinson’s disease: two-year follow-up. Neurology 58:396–401
Levy G, Tang MX, Cote LJ, Louis ED, Alfaro B, Mejia H, Stern Y, Marder K (2000) Motor impairment in PD: relationship to incident dementia and age. Neurology 55:539–544
Abbruzzese G, Berardelli A (2003) Sensorimotor integration in movement disorders. Mov Disord 18:231–240
Zia S, Cody F, O’Boyle D (2000) Joint position sense is impaired by Parkinson’s disease. Ann Neurol 47:218–228
Rickards C, Cody FW (1997) Proprioceptive control of wrist movements in Parkinson’s disease. Reduced muscle vibration-induced errors. Brain 120:977–990
Schneider JS, Diamond SG, Markham CH (1986) Deficits in orofacial sensorimotor function in Parkinson’s disease. Ann Neurol 19:275–282
Artieda J, Pastor MA, Lacruz F, Obeso JA (1992) Temporal discrimination is abnormal in Parkinson’s disease. Brain 115:199–210
Klockgether T, Borutta M, Rapp H, Spieker S, Dichgans J (1995) A defect of kinesthesia in Parkinson’s disease. Mov Disord 10:460–465
Berardelli A, Dick JP, Rothwell JC, Day BL, Marsden CD (1986) Scaling of the size of the first agonist EMG burst during rapid wrist movements in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry 49:1273–1279
Demirci M, Grill S, McShane L, Hallett M (1997) A mismatch between kinesthetic and visual perception in Parkinson’s disease. Ann Neurol 41:781–788
Berardelli A, Rothwell JC, Thompson PD, Hallett M (2001) Pathophysiology of bradykinesia in Parkinson’s disease. Brain 124:2131–2146
Rocchi L, Chiari L, Mancini M, Carlson-Kuhta P, Gross A, Horak FB (2006) Step initiation in Parkinson’s disease: influence of initial stance conditions. Neurosci Lett 406:128–132
Lyoo CH, Aalto S, Rinne JO, Lee KO, Oh SH, Chang JW, Lee MS (2007) Different cerebral cortical areas influence the effect of subthalamic nucleus stimulation on parkinsonian motor deficits and freezing of gait. Mov Disord 22:2176–2182
Patchay S, Gahery Y (2003) Effect of asymmetrical limb loading on early postural adjustments associated with gait initiation in young healthy adults. Gait Posture 18:85–94
Burleigh-Jacobs A, Horak FB, Nutt JG, Obeso JA (1997) Step initiation in Parkinson’s disease: influence of levodopa and external sensory triggers. Mov Disord 12:206–215
Jacobs JV, Horak FB (2006) Abnormal proprioceptive-motor integration contributes to hypometric postural responses of subjects with Parkinson’s disease. Neuroscience 141:999–1009
Wright WG, Gurfinkel VS, King LA, Nutt JG, Cordo PJ, Horak FB (2010) Axial kinesthesia is impaired in Parkinson’s disease: effects of levodopa. Exp Neurol 225:202–209
Konczak J, Corcos DM, Horak F, Poizner H, Shapiro M, Tuite P, Volkmann J, Maschke M (2009) Proprioception and motor control in Parkinson’s disease. J Mot Behav 41:543–552
Lee MS, Kim HS, Lyoo CH (2005) “Off” gait freezing and temporal discrimination threshold in patients with Parkinson disease. Neurology 64:670–674
Camicioli R, Gee M, Bouchard TP, Fisher NJ, Hanstock CC, Emery DJ, Martin WR (2009) Voxel-based morphometry reveals extra-nigral atrophy patterns associated with dopamine refractory cognitive and motor impairment in parkinsonism. Parkinsonism Relat Disord 15:187–195
Fogassi L, Luppino G (2005) Motor functions of the parietal lobe. Curr Opin Neurobiol 15:626–631
Rizzolatti G, Luppino G, Matelli M (1998) The organization of the cortical motor system: new concepts. Electroencephalogr Clin Neurophysiol 106:283–296
Andersen RA, Buneo CA (2002) Intentional maps in posterior parietal cortex. Annu Rev Neurosci 25:189–220
Helmich RC, Derikx LC, Bakker M, Scheeringa R, Bloem BR, Toni I (2010) Spatial remapping of cortico-striatal connectivity in Parkinson’s disease. Cereb Cortex 20:1175–1186
Hinkley LB, Krubitzer LA, Nagarajan SS, Disbrow EA (2007) Sensorimotor integration in S2, PV, and parietal rostroventral areas of the human sylvian fissure. J Neurophysiol 97:1288–1297
Louis ED, Tang MX, Cote L, Alfaro B, Mejia H, Marder K (1999) Progression of parkinsonian signs in Parkinson disease. Arch Neurol 56:334–337
Pirker W (2003) Correlation of dopamine transporter imaging with parkinsonian motor handicap: how close is it? Mov Disord 18(Suppl 7):S43–S51
Benamer HT, Patterson J, Wyper DJ, Hadley DM, Macphee GJ, Grosset DG (2000) Correlation of Parkinson’s disease severity and duration with 123I-FP-CIT SPECT striatal uptake. Mov Disord 15:692–698
Lewis MM, Du G, Sen S, Kawaguchi A, Truong Y, Lee S, Mailman RB, Huang X (2011) Differential involvement of striato- and cerebello-thalamo-cortical pathways in tremor- and akinetic/rigid-predominant Parkinson’s disease. Neuroscience 177:230–239
Rodriguez-Oroz MC, Jahanshahi M, Krack P, Litvan I, Macias R, Bezard E, Obeso JA (2009) Initial clinical manifestations of Parkinson’s disease: features and pathophysiological mechanisms. Lancet Neurol 8:1128–1139
Acknowledgments
This work was supported by a faculty research grant of Yonsei University College of Medicine (grant number 6-2010-0016).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lyoo, C.H., Ryu, Y.H. & Lee, M.S. Cerebral cortical areas in which thickness correlates with severity of motor deficits of Parkinson’s disease. J Neurol 258, 1871–1876 (2011). https://doi.org/10.1007/s00415-011-6045-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-011-6045-6