Abstract
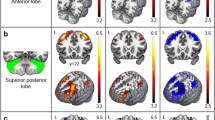
Friedreich ataxia (FRDA) is traditionally associated with neuropathology in the cerebellar dentate nucleus and spinal cord. Growing evidence also suggests involvement of the cerebral and cerebellar cortices, although reports of structural abnormalities remain mixed. This study assessed the structural integrity of cortical grey matter in FRDA, focussing on regions in which pathology may underlie the motor deficits characteristic of this disorder. T1-weighted anatomical magnetic resonance imaging scans were acquired from 31 individuals with FRDA and 37 healthy controls. Cortical thickness (FreeSurfer) and cortical volume (SPM-VBM) were measured in cerebral motor regions-of-interest (primary motor, dorsal and ventral premotor, and supplementary motor areas) alongside unconstrained exploratory analyses of the cerebral and cerebellar cortices. Correlations were assessed between cortical thickness/volume measures and each of disease severity, length of the causative genetic triplet-repeat expansion, and finger-tapping behavioural measures. Individuals with FRDA had significantly reduced cortical thickness, relative to controls, in the premotor and supplementary motor areas. Reduced cortical thickness and/or volume were also observed in the cuneus and precuneus, posterior aspects of the medial and lateral prefrontal cortices, insula, temporal poles, and cerebellar lobules V, VI, and VII. Measures of clinical severity, genetic abnormality, and motor dysfunction correlated with volume loss in the lateral cerebellar hemispheres. These results suggest that atrophy preferentially affects premotor relative to primary areas of the cortical motor system, and also extends to a range of non-motor brain regions. Furthermore, cortical thickness and cortical volume findings were largely divergent, suggesting that each is sensitive to different aspects of neuropathology in FRDA. Overall, this study supports a disease model involving neural aberrations within the cerebral and cerebellar cortices, beyond those traditionally associated with this disorder.



Similar content being viewed by others
References
Akhlaghi H, Corben L, Georgiou-Karistianis N, Bradshaw J, Delatycki MB, Storey E, Egan GF (2012) A functional MRI study of motor dysfunction in Friedreich’s ataxia. Brain Res 1471:138–154
Akhlaghi H, Yu J, Corben L, Georgiou-Karistianis N, Bradshaw JL, Storey E, Delatycki MB, Egan GF (2014) Cognitive deficits in Friedreich ataxia correlate with micro-structural changes in dentatorubral tract. Cerebellum 13:187–198
Ashburner J, Friston KJ (2000) Voxel-based morphometry—the methods. NeuroImage 11:805–821
Barulli D, Stern Y (2013) Efficiency, capacity, compensation, maintenance, plasticity: emerging concepts in cognitive reserve. Trends Cogn Sci 17:502–509. doi:10.1016/j.tics.2013.1008.1012
Beck AT, Steer RA, Brown GK (1996) Manual for the BDI-II. The Psychological Corporation, San Antonio
Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BTT (2011) The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol 106:2322–2345
Carrera E, Tononi G (2014) Diaschisis: past, present, future. Brain J Neurol 137:2408–2422
Corben LA, Delatycki MB, Bradshaw JL, Churchyard AJ, Georgiou-Karistianis N (2011) Utilisation of advance motor information is impaired in Friedreich ataxia. Cerebellum 10:793–803
Della Nave R, Ginestroni A, Giannelli M, Tessa C, Salvatore E, Salvi F, Dotti MT, De Michele G, Piacentini S, Mascalchi M (2008) Brain structural damage in Friedreich’s ataxia. J Neurol Neurosurg Psychiatry 79:82–85
Della Nave R, Ginestroni A, Tessa C, Salvatore E, Bartolomei I, Salvi F, Dotti MT, De Michele G, Piacentini S, Mascalchi M (2008) Brain white matter tracts degeneration in Friedreich ataxia. An in vivo MRI study using tract-based spatial statistics and voxel-based morphometry. NeuroImage 40:19–25
Fischl B, Dale AM (2000) Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci 97:11050–11055
Fornito A, Zalesky A, Breakspear M (2015) The connectomics of brain disorders. Nat Rev Neurosci 16:159–172
Franca MC Jr, D’Abreu A, Yasuda CL, Bonadia LC, Santos da Silva M, Nucci A, Lopes-Cendes I, Cendes F (2009) A combined voxel-based morphometry and 1H-MRS study in patients with Friedreich’s ataxia. J Neurol 256:1114–1120
Georgiou-Karistianis N, Akhlaghi H, Corben LA, Delatycki MB, Storey E, Bradshaw JL, Egan GF (2012) Decreased functional brain activation in Friedreich ataxia using the Simon effect task. Brain Cogn 79:200–208
Ginestroni A, Diciotti S, Cecchi P, Pesaresi I, Tessa C, Giannelli M, Nave RD, Salvatore E, Salvi F, Dotti MT, Piacentini S, Soricelli A, Cosottini M, De Stefano N, Mascalchi M (2012) Neurodegeneration in Friedreich’s ataxia is associated with a mixed activation pattern of the brain. A fMRI study. Hum Brain Mapp 33:1780–1791
Hagler DJ Jr, Saygin AP, Sereno MI (2006) Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. NeuroImage 33:1093–1103
Harding IH, Corben LA, Storey E, Egan GF, Stagnitti MR, Poudel G, Delatycki MB, Georgiou-Karistianis N (2016) Fronto-cerebellar dysfunction and dysconnectivity underlying cognitive functioning in Friedreich ataxia: the IMAGE-FRDA study. Hum Brain Mapp 37:338–350
Harding IH, Raniga P, Delatycki MB, Stagnitti MR, Corben LA, Storey E, Georgiou-Karistianis N, Egan GF (2016) Tissue atrophy and elevated iron concentration in the extrapyramidal motor system in Friedreich ataxia: the IMAGE-FRDA study. J Neurol Neurosurg Psychiatry. doi:10.1136/jnnp-2015-312665
Hernandez-Castillo CR, Galvez V, Diaz R, Fernandez-Ruiz J (2016) Specific cerebellar and cortical degeneration correlates with ataxia severity in spinocerebellar ataxia type 7. Brain Imaging Behav 10:252–257
Hocking DR, Corben LA, Fielding J, Cremer PD, Millist L, White OB, Delatycki MB (2014) Saccade reprogramming in Friedreich ataxia reveals impairments in the cognitive control of saccadic eye movement. Brain Cogn 87:161–167
Hwang K, Hallquist MN, Luna B (2012) The development of hub architecture in the human functional brain network. Cereb Cortex 23:2380–2393
Im K, Lee J-M, Lyttelton O, Kim SH, Evans AC, Kim SI (2008) Brain size and cortical structure in the adult human brain. Cereb Cortex 18:2181–2191
Koeppen AH, Davis AN, Morral JA (2011) The cerebellar component of Friedreich’s ataxia. Acta Neuropathol 122:323–330
Koeppen AH, Mazurkiewicz JE (2013) Friedreich ataxia: neuropathology revised. J Neuropathol Exp Neurol 72:78–90
Kong L, Herold CJ, Zöllner F, Salat DH, Lässer MM, Schmid LA, Fellhauer I, Thomann PA, Essig M, Schad LR, Erickson KI, Schröder J (2015) Comparison of grey matter volume and thickness for analysing cortical changes in chronic schizophrenia: a matter of surface area, grey/white matter intensity contrast, and curvature. Psychiatry Res Neuroimaging 231:176–183
Mayka MA, Corcos DM, Leurgans SE, Vaillancourt DE (2006) Three-dimensional locations and boundaries of motor and premotor cortices as defined by functional brain imaging: a meta-analysis. NeuroImage 31:1453–1474
Menon V, Uddin LQ (2010) Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 214:655–667
Nachbauer W, Bodner T, Boesch S, Karner E, Eigentler A, Neier L, Benke T, Delazer M (2014) Friedreich ataxia: executive control is related to disease onset and GAA repeat length. Cerebellum 13:9–16
Nieto A, Correia R, de Nobrega E, Montón F, Hess S, Barroso J (2012) Cognition in Friedreich ataxia. Cerebellum 11:834–844
Pandolfo M (2009) Friedreich ataxia: the clinical picture. J Neurol 256:3–8
Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, Jacobson K, Lyons MJ, Grant MD, Franz CE, Xian H, Tsuang M, Fischl B, Seidman L, Dale A, Kremen WS (2009) Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex (New York, NY 19:2728–2735
Rezende TJR, Silva CB, Yassuda CL, Campos BM, D’Abreu A, Cendes F, Lopes-Cendes I, França MC (2016) Longitudinal magnetic resonance imaging study shows progressive pyramidal and callosal damage in Friedreich’s ataxia. Mov Disord 31:70–78
Sanabria-Diaz G, Melie-García L, Iturria-Medina Y, Alemán-Gómez Y, Hernández-González G, Valdés-Urrutia L, Galán L, Valdés-Sosa P (2010) Surface area and cortical thickness descriptors reveal different attributes of the structural human brain networks. NeuroImage 50:1497–1510
Schulz JB, Borkert J, Wolf S, Schmitz-Hübsch T, Rakowicz M, Mariotti C, Schoels L, Timmann D, van de Warrenburg B, Dürr A, Pandolfo M, Kang J-S, Mandly AG, Nägele T, Grisoli M, Boguslawska R, Bauer P, Klockgether T, Hauser T-K (2010) Visualization, quantification and correlation of brain atrophy with clinical symptoms in spinocerebellar ataxia types 1, 3 and 6. NeuroImage 49:158–168
Silk TJ, Beare R, Malpas C, Adamson C, Vilgis V, Vance A, Bellgrove MA (2016) Cortical morphometry in attention deficit/hyperactivity disorder: contribution of thickness and surface area to volume. Cortex 82:1–10
Smith SM, Nichols TE (2009) Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage 44:83–98
Sporns O, Honey CJ, Kötter R (2007) Identification and classification of hubs in brain networks. PLoS One 2:e1049
Stout JC, Paulsen JS, Queller S, Solomon AC, Whitlock KB, Campbell JC, Carlozzi N, Duff K, Beglinger LJ, Langbehn DR, Johnson SA, Biglan KM, Aylward EH (2011) Neurocognitive signs in prodromal Huntington disease. Neuropsychology 25:1–14
Subramony SH, May W, Lynch D, Gomez C, Fischbeck K, Hallett M, Taylor P, Wilson R, Ashizawa T (2005) Measuring Friedreich ataxia: interrater reliability of a neurologic rating scale. Neurology 64:1261–1262
Tomasi D, Volkow ND (2011) Functional connectivity hubs in the human brain. NeuroImage 57:908–917
van den Heuvel MP, Sporns O (2013) Network hubs in the human brain. Trends Cognit Sci 17:683–696
Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, Duggirala R, Glahn DC (2010) Cortical thickness or grey matter volume? the importance of selecting the phenotype for imaging genetics studies. NeuroImage 53:1135–1146
Yang J-J, Kwon H, Lee J-M (2016) Complementary characteristics of correlation patterns in morphometric correlation networks of cortical thickness, surface area, and gray matter volume. Sci Reports 6:26682
Zalesky A, Akhlaghi H, Corben LA, Bradshaw JL, Delatycki MB, Storey E, Georgiou-Karistianis N, Egan GF (2014) Cerebello-cerebral connectivity deficits in Friedreich ataxia. Brain Struct Funct 219:969–981
Acknowledgments
Funding support was provided by the Australian National Health and Medical Research Council (Project Grant 1046037). We thank Dr. Dominic Dwyer and Dr. Sarah Whittle for technical consultations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
L. P. Selvadurai and I. H. Harding contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Selvadurai, L.P., Harding, I.H., Corben, L.A. et al. Cerebral and cerebellar grey matter atrophy in Friedreich ataxia: the IMAGE-FRDA study. J Neurol 263, 2215–2223 (2016). https://doi.org/10.1007/s00415-016-8252-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-016-8252-7