Abstract
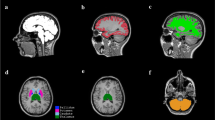
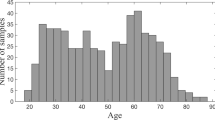
The objective is to estimate average global and regional percentage brain volume loss per year (BVL/year) of the physiologically ageing brain. Two independent, cross-sectional single scanner cohorts of healthy subjects were included. The first cohort (n = 248) was acquired at the Medical Prevention Center (MPCH) in Hamburg, Germany. The second cohort (n = 316) was taken from the Open Access Series of Imaging Studies (OASIS). Brain parenchyma (BP), grey matter (GM), white matter (WM), corpus callosum (CC), and thalamus volumes were calculated. A non-parametric technique was applied to fit the resulting age–volume data. For each age, the BVL/year was derived from the age–volume curves. The resulting BVL/year curves were compared between the two cohorts. For the MPCH cohort, the BVL/year curve of the BP was an increasing function starting from 0.20% at the age of 35 years increasing to 0.52% at 70 years (corresponding values for GM ranged from 0.32 to 0.55%, WM from 0.02 to 0.47%, CC from 0.07 to 0.48%, and thalamus from 0.25 to 0.54%). Mean absolute difference between BVL/year trajectories across the age range of 35–70 years was 0.02% for BP, 0.04% for GM, 0.04% for WM, 0.11% for CC, and 0.02% for the thalamus. Physiological BVL/year rates were remarkably consistent between the two cohorts and independent from the scanner applied. Average BVL/year was clearly age and compartment dependent. These results need to be taken into account when defining cut-off values for pathological annual brain volume loss in disease models, such as multiple sclerosis.


Similar content being viewed by others
References
Barkhof F et al (2009) Imaging outcomes for neuroprotection and repair in multiple sclerosis trials. Nat Rev Neurol 5(5):256–266
Steenwijk MD et al (2016) Cortical atrophy patterns in multiple sclerosis are non-random and clinically relevant. Brain 139(Pt 1):115–126
Fisher E et al (2008) Gray matter atrophy in multiple sclerosis: a longitudinal study. Ann Neurol 64(3):255–265
Sepulcre J et al (2006) Regional gray matter atrophy in early primary progressive multiple sclerosis: a voxel-based morphometry study. Arch Neurol 63(8):1175–1180
Audoin B et al (2006) Localization of grey matter atrophy in early RRMS: a longitudinal study. J Neurol 253(11):1495–1501
Datta S et al (2015) Regional gray matter atrophy in relapsing remitting multiple sclerosis: baseline analysis of multi-center data. Mult Scler Relat Disord 4(2):124–136
Zivadinov R et al (2013) Evolution of cortical and thalamus atrophy and disability progression in early relapsing-remitting MS during 5 years. AJNR Am J Neuroradiol 34(10):1931–1939
Rocca MA et al (2010) Thalamic damage and long-term progression of disability in multiple sclerosis. Radiology 257(2):463–469
Schoonheim MM et al (2012) Subcortical atrophy and cognition: sex effects in multiple sclerosis. Neurology 79(17):1754–1761
Pelletier J et al (2001) A longitudinal study of callosal atrophy and interhemispheric dysfunction in relapsing-remitting multiple sclerosis. Arch Neurol 58:105–111
Uher T et al (2016) Combining clinical and magnetic resonance imaging markers enhances prediction of 12-year disability in multiple sclerosis. Mult Scler. doi:10.1177/1352458516642314
De Stefano N et al (2016) Establishing pathological cut-offs of brain atrophy rates in multiple sclerosis. J Neurol Neurosurg Psychiatry 87(1):93–99
Ziegler G et al (2012) Brain structural trajectories over the adult lifespan. Hum Brain Mapp 33(10):2377–2389
Marcus DS et al (2007) Open access series of imaging studies (OASIS): cross-sectional MRI data in young, middle aged, nondemented, and demented older adults. J Cogn Neurosci 19(9):1498–1507
Ge Y et al (2002) Age-related total gray matter and white matter changes in normal adult brain. Part I: volumetric MR imaging analysis. AJNR Am J Neuroradiol 23(8):1327–1333
Fotenos AF et al (2005) Normative estimates of cross-sectional and longitudinal brain volume decline in aging and AD. Neurology 64(6):1032–1039
Fjell AM et al (2013) Critical ages in the life course of the adult brain: nonlinear subcortical aging. Neurobiol Aging 34(10):2239–2247
Marcus DS et al (2010) Open access series of imaging studies: longitudinal MRI data in nondemented and demented older adults. J Cogn Neurosci 22(12):2677–2684
Hedman AM et al (2012) Human brain changes across the life span: a review of 56 longitudinal magnetic resonance imaging studies. Hum Brain Mapp 33(8):1987–2002
Enzinger C et al (2005) Risk factors for progression of brain atrophy in aging: six-year follow-up of normal subjects. Neurology 64(10):1704–1711
Opfer R et al (2016) Atlas based brain volumetry: how to distinguish regional volume changes due to biological or physiological effects from inherent noise of the methodology. Magn Reson Imaging 34(4):455–461
Huppertz HJ et al (2010) Intra- and interscanner variability of automated voxel-based volumetry based on a 3D probabilistic atlas of human cerebral structures. Neuroimage 49(3):2216–2224
Ashburner J, Friston KJ (2005) Unified segmentation. Neuroimage 26(3):839–851
Ashburner J (2007) A fast diffeomorphic image registration algorithm. Neuroimage 38(1):95–113
Mori S et al (2008) Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 40(2):570–582
Malone IB et al (2015) Accurate automatic estimation of total intracranial volume: a nuisance variable with less nuisance. Neuroimage 104:366–372
Keihaninejad S et al (2010) A robust method to estimate the intracranial volume across MRI field strengths (1.5T and 3T). Neuroimage 50(4):1427–1437
Pell GS et al (2008) Selection of the control group for VBM analysis: influence of covariates, matching and sample size. Neuroimage 41(4):1324–1335
Friedman J, Hastie T, Tibshirani R (2001) The elements of statistical learning (chapter 6), vol 1. Springer Series in Statistics, Springer, Berlin
Bowman AW, Azzalini A (1997) Applied smoothing techniques for data analysis: the kernel approach with S-Plus illustrations: the kernel approach with S-Plus illustrations. OUP, Oxford
Fjell AM et al (2013) Brain changes in older adults at very low risk for Alzheimer’s disease. J Neurosci 33(19):8237–8242
De Stefano N et al (2010) Assessing brain atrophy rates in a large population of untreated multiple sclerosis subtypes. Neurology 74(23):1868–1876
Filippi M et al (2014) Placebo-controlled trial of oral laquinimod in multiple sclerosis: MRI evidence of an effect on brain tissue damage. J Neurol Neurosurg Psychiatry 85(8):851–858
Pfefferbaum A et al (2013) Variation in longitudinal trajectories of regional brain volumes of healthy men and women (ages 10 to 85 years) measured with atlas-based parcellation of MRI. Neuroimage 65:176–193
Pfefferbaum A, Sullivan EV (2015) Cross-sectional versus longitudinal estimates of age-related changes in the adult brain: overlaps and discrepancies. Neurobiol Aging 36(9):2563–2567
Lindenberger U et al (2011) Cross-sectional age variance extraction: what’s change got to do with it? Psychol Aging 26(1):34–47
Gordon BA et al (2008) Neuroanatomical correlates of aging, cardiopulmonary fitness level, and education. Psychophysiology 45(5):825–838
Noble KG et al (2012) Hippocampal volume varies with educational attainment across the life-span. Front Hum Neurosci 6:307
Foubert-Samier A et al (2012) Education, occupation, leisure activities, and brain reserve: a population-based study. Neurobiol Aging 33(2):423.e15–25
Fjell AM et al (2010) When does brain aging accelerate? Dangers of quadratic fits in cross-sectional studies. Neuroimage 50(4):1376–1383
Acknowledgements
The OASIS database is made available by the Washington University Alzheimer’s Disease Research Center, Dr. Randy Buckner at the Howard Hughes Medical Institute (HHMI) at Harvard University, the Neuroinformatics Research Group (NRG) at Washington University School of Medicine, and the Biomedical Informatics Research Network (BIRN), and supported by NIH grants P50 AG05681, P01 AG03991, R01 AG021910, P50 MH071616, U24 RR021382, R01 MH56584.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical standard
The study (MPCH cohort) was approved by the Ethics Board of the Ärztekammer, Hamburg, Germany.
Informed consent
All patients gave written informed consent.
Electronic supplementary material
Below is the link to the electronic supplementary material.
415_2016_8374_MOESM1_ESM.tiff
Supplementary material 1 (TIFF 264 kb) Fig. 3 Two plots show simulated age–volume trajectories. The trajectories are modelled by a set of quadratic functions \({\text{g}}_{\text{i}} \left( {\text{x}} \right) = {\text{a}}_{\text{i}} {\text{x}}^{2} + {\text{b}}_{\text{i}} {\text{x}} + {\text{c}}_{\text{i}}\) with some random coefficients \({\text{a}}_{\text{i}} ,{\text{b}}_{\text{i}} ,{\text{c}}_{\text{i}}\). The range of the coefficients is estimated from our data. The left plot shows 300 random trajectories and the mean trajectory (black curve). In the right plot, one single timepoint on each trajectory was randomly selected (grey dots). Based on these given single timepoints, the true mean trajectory (black curve) was estimated with the non-parametric technique described in the manuscript (dashed line). We can observe that the “true” mean curve is reasonably close to the estimated curve (dashed line)
Rights and permissions
About this article
Cite this article
Schippling, S., Ostwaldt, AC., Suppa, P. et al. Global and regional annual brain volume loss rates in physiological aging. J Neurol 264, 520–528 (2017). https://doi.org/10.1007/s00415-016-8374-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-016-8374-y