Abstract
Background
Because of their fragile and thin wall, ruptured blood blister-like aneurysms (BBAs) at the anterior wall of the internal carotid artery (ICA) are difficult to manage, both surgically, as well as endovascularly. BBA is usually a tiny and broad-necked aneurysm, but it occasionally demonstrates a relatively saccular-like shape. In addition, the pseudoaneurysm sac often assumes a saccular shape. In this paper, the authors present their experience in treating these saccular-shaped BBAs endovascularly with coil packing.
Method
Nine saccular-shaped ruptured BBAs in nine patients (one male and eight females; mean age 51.3 years, range 38–76) were treated with coil packing of the lesion between January 2006 and August 2010 in Nagoya University and its affiliated hospitals. Clinical, procedural, and angiographic data were retrospectively evaluated.
Findings
Seven BBAs were treated by balloon-assisted coil embolization. Two remaining BBAs were embolized without balloon inflation, though a balloon catheter was on standby at the ICA. In one case, in which a saccular coil embolization could not be achieved, ICA trapping was performed. Three (33.3%) were treated in acute, two (22.2%) in subacute, and four (44.4%) in chronic period. One (11.1%) intraoperative rupture occurred. Six (66.7%) had excellent clinical outcomes, while two (22.2%) proved fatal outcomes. During the follow-up period (mean 18.9 months, range 4–48), two out of seven (28.6%) aneurysms presented an angiographical recurrence, but both were treated by coil embolization without complications. The remaining five (71.4%) aneurysms were completely resolved.
Conclusions
Endovascular coil embolization can be considered as an alternative treatment option for selective saccular-shaped BBAs.
Similar content being viewed by others
Introduction
Blood blister-like aneurysms (BBAs) at the anterior wall of the internal carotid artery (ICA) are rare causes of acute subarachnoid hemorrhage (SAH) but known to be quite strange and dangerous aneurysms by neurosurgeons. Because of their fragile and thin wall, ruptured BBAs are difficult to manage both surgically, as well as endovascularly [15, 18]. In addition, they are often associated with high morbidity and mortality rates. While BBA is usually a tiny and broad-necked aneurysm, such an aneurysm occasionally demonstrates a relatively saccular-like shape on an angiogram. Additionally, the pseudoaneurysm sac often assumes a saccular shape.
In this paper, the authors present their experience in treating these saccular-shaped BBAs endovascularly with coil packing.
Methods and materials
Patient population
The authors reviewed the records of all patients with ICA BBAs who presented with SAH who underwent endovascular treatment between January 2006 and August 2010 in Nagoya University Hospital and its affiliated hospitals. Nine saccular-shaped ruptured BBAs in nine patients (one male and eight females; mean age of 51.3 years, range 38–76) were treated with coil packing of the saccular cavity. Those patients were diagnosed as ruptured BBAs by typical angiographical findings, or any rapid configurational change in the aneurysm. Aneurysms that appeared to be authentic saccular types [18, 21] were excluded. Clinical features, treatment methods, procedure-related complications, and clinical and angiographic outcomes were retrospectively evaluated.
Clinical and angiographic follow-up
Using the Hunt and Hess grading system, initial clinical assessments were performed on admission. Clinical outcomes were evaluated at discharge or clinical follow-ups according to their Glasgow Outcome Scale (GOS) scores. GOS indicates score 1: good recovery (GR), 2: moderate disability (MD), 3: severe disability (SD), 4: vegetative state (VS), and 5: death (D). Each patient's clinical status at the last follow-up was defined as the final outcome.
An angiographic follow-up was performed at least once before discharge. After discharge, an MR angiogram (MRA) and/or X-ray imaging study were performed once a week for about 1 month, and once every 2–4 weeks for 3 months, postoperatively. A subsequent follow-up interval was scheduled, depending on the interventionalists' requirement. Whenever these images indicated an aneurysm recurrence, an angiogram was considered for minute investigation.
Endovascular treatment
Neurosurgeons and interventionalists have thoroughly discussed the issue of treatment strategy. Endovascular coil embolizations were chosen after considering angiographical images, patient clinical condition, and the procedural risks of complication compared to other treatment options.
All procedures were conducted with the patient in a state of general anesthesia or deep sedation. A 6-Fr sheath was inserted at the femoral artery, and a 6-Fr guiding catheter was placed at the ICA. A 10-type microcatheter was meticulously navigated to the aneurysm with a microwire guide. A balloon microcatheter was navigated to the ICA C1–C2 portion where the BBA neck was located.
Detachable coils were carefully inserted into the cavity and deployed in the usual manner. When the coil loops easily protruded into an ICA and were not compactly placed within the sac, they were deployed with balloon assistance. However, the balloon was inflated as moderately as possible, given that ICA BBAs were potentially fragile and posed a risk of rupture. Softer coils were preferred in coil selection. No stent-assisted coil embolization was performed, since intracranial stents were not then available in Japan.
Heparin was usually not given either during or after the procedure, nor was antiplatelet therapy administered during the periprocedural period.
Results
A clinical and interventional summary is presented in Table 1. Patients' Hunt and Hess grades were II in 1, III in 4, IV in 3 and V in 1. Three patients were treated in an acute period (0–3 days after onset), two in a subacute period (6–13 days after onset), and four in a chronic period (after 14 days after onset). In both subacute cases, initial angiograms did not clearly show the aneurysm, whereas repeated angiograms obtained in the subacute period disclosed the BBA. Therefore, treatment was performed in the subacute period. Seven BBAs were treated by balloon-assisted coil embolization, while the two remaining BBAs were embolized without balloon inflation, though a balloon catheter was on standby at the ICA.
In case 8, saccular coil embolization was initially planned for the early period. However, the detachable coils were not compactly stabilized inside the pseudoaneurysm sac and readily protruded into the ICA despite the balloon assistance, which resulted in aneurysm trapping with parent artery occlusion. ICA trapping was successfully performed while preserving the posterior communicating artery (PcomA) and the anterior choroidal artery (AchoA). However, postoperatively, severe ischemia was induced by initial brain damage and subsequent vasospasm. Thus, a large hemispheric infarction developed, and the patient died 13 days after the procedure.
One (11.1%) intraoperative rupture occurred during coil insertion into the aneurysm sac (case 6). In that case, recourse to balloon inflation immediately after recognizing the extravasation of contrast medium and further coil deposition, resulted in terminating the bleeding. Post-procedural computed tomography (CT) revealed a small increase of SAH, and the patient's clinical condition slightly deteriorated. As a result, though the patient gradually recovered in the following months, she still suffers a minor motor deficit, as well as moderate neuropsychological impairment (GOS score, 2). In that case, no aneurysm recurrence or rebleeding was observed in the follow-up study.
Six patients (66.7%) had excellent clinical outcomes (GOS score, 1) and two (22.2%) had fatal outcomes (GOS score, 5).
During the follow-up period (mean 18.9 months, range 4–48), two out of seven (28.6%) aneurysms that presented with angiographical recurrence were both treated with saccular coil embolization (cases 2 and 3). Those recurrences emerged within several weeks after embolization without rebleeding. The remaining four aneurysms (71.4%) were completely resolved during the follow-up period.
Illustrative cases
Case 2
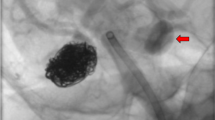
A 50-year-old woman visited the emergency department, complaining of severe sudden onset of headaches, and a CT scan revealed diffuse SAH. She was diagnosed as a Hunt and Hess grade III-SAH. However, the angiogram carried out on the next day demonstrated neither any apparent intracranial aneurysm nor other bleeding source. (Retrospectively, a small irregularity at the anterior wall on the right ICA was suspected) (Fig. 1a). The patient was, therefore, treated conservatively. Repeated angiogram obtained 10 days after onset revealed a right ICA aneurysm that showed a saccular-like shape (Fig. 1b). Therefore, coil embolization was performed with balloon assistance. A total of three coils were inserted into the aneurysm cavity (Fig. 1c). No procedural complications occurred. The patient was discharged without any neurological deficit. The follow-up angiogram obtained 4 weeks later demonstrated an aneurysm recurrence (Fig. 1d). The aneurysm neck was enlarged, and the coil mass was compressed. A second embolization uneventfully resolved this recurrence (Fig. 1e). During the following 9 months, re-recurrence of the aneurysm gradually developed (Fig. 1f). This time, a third embolization was done without complications (Fig. 1g , h). After 2 years, no aneurysm recurrence has been observed. The patient has shown no neurological deficit, and her clinical outcome is excellent (GOS score, 1).
Case 2. a Left ICA angiogram acquired the day after symptom onset demonstrating a suspicious small irregularity at the anterior wall on the ICA (arrow). b Repeated angiogram 10 days later disclosing a saccular-shaped BBA of the ICA. c Angiogram acquired after endosaccular embolization by a balloon-assisted technique, showing near complete occlusion of the aneurysm. d Angiogram 1 month later, demonstrating coil compaction and regrowth of the aneurysm (arrow). e Angiogram obtained after second embolization. f Angiogram 9 months later, revealing regrowth of the aneurysm neck (arrow). g, h Angiogram obtained after third embolization (g non-subtraction image, h subtraction image) demonstrating complete occlusion of the BBA
In this case, the fact that initially coiling of only the pseudoaneurysm sac, which had no normal wall corresponding to the dissected part of the ICA, could not dispose of the lesion completely, and could not prevent the aneurysm recurrence. However, it might prevent the fatal rebleeding, and finally, after the repeated embolizations, the lesion was entirely resolved.
Case 7
A 39-year-old man presented with a Hunt and Hess grade III-SAH. Cerebral angiogram demonstrated a broad-based bulge on the anterior wall of the right distal ICA (Fig. 2a, b). The patient underwent a right frontotemporal craniotomy and wrapping of this unclippable BBA without an intraprocedural rupture. On postoperative day 10, MRA revealed a remarkable configurational change in the aneurysm with a rapid growth of the pseudoaneurysm sac (Fig. 2c, d). In this patient, both PcomA and AchoA originated so close to the aneurysm that ICA trapping was believed to be impossible. Since his aneurysm seemed to be amenable to endovascular coiling, he was then treated by coil embolization of the saccular pseudoaneurysm sac on postoperative day 14. A total of four coils were placed inside the sac with balloon assistance (Fig. 2e, f), and no complications (including an aneurysm rerupture) occurred during the procedure. The patient recovered well at discharge and returned to his usual job despite a very mild neuropsychological impairment (GOS score, 1). Follow-up MRAs obtained 3 and 16 weeks later demonstrated no aneurysm recurrence (Fig. 2g, h). The anterior wall of the ICA was found to have become smooth at a 16-week MRA (Fig. 2h).
Case 7. a Right ICA angiogram acquired the day after symptom onset demonstrating a BBA; broad-based bulge on the anterior wall of the right distal ICA. b Initial three-dimensional (3D) reconstruction angiogram also showing the BBA of the ICA. c, d (c conventional angiogram, d 3D angiogram): Angiogram obtained 14 days after wrapping that discloses a remarkable configurational change in the aneurysm with a rapid growth of the pseudoaneurysm sac (arrow). e, f: Angiogram acquired after coil embolization by a balloon-assisted technique, showing complete occlusion of the pseudoaneurysm sac. g, h MRA performed 3 and 16 weeks after embolization, respectively, demonstrating no regrowth of the aneurysm. Anterior wall of the ICA was found to have become smooth at a 16-week MRA (arrow) (h)
In this case, packing of the aneurysm cavity might achieve the stabilization of the weak part of the lesion without full exclusion of the vessel wall itself. That effect helped the spontaneous remodeling of the affected ICA.
Discussion
Clinical features of BBAs
Internal carotid artery BBAs are characterized by extremely fragile and thin walls that make therapeutic management difficult and hazardous, both surgically, as well as endovascularly [15, 18]. BBAs exclusively arise at non-branching sites of the supraclinoidal ICA, and are variously referred to as “dorsal wall” [21] and “anterior wall” ICA aneurysms [20], or “ICA trunk aneurysm” [18]. BBAs usually present various typical angiographical findings (e.g., tiny hemispheric bleb, broad-based bulge, irregular protrusion of the anterior wall of the ICA, and rapid configurational change or regrowth). In our series, ruptured BBA patients were diagnosed based on these findings. Aneurysms that appeared to be authentic saccular type, which could be considered ordinary aneurysms with an obviously firm neck and a saccular dome, were excluded in order not to confuse the matter. The pathogenesis of BBAs remains unclear. Several studies suggest that BBA may not be a true saccular aneurysm, but rather a specific type of pseudoaneurysm or dissecting aneurysm [7, 21]. Ishikawa and his colleagues reported in their article that BBA appears to be a laceration of the carotid wall based on degeneration of the internal elastic lamina [7]. Hemodynamic stress probably plays an important role in the formation of BBAs because the anterior wall of the ICA is curved, where the flux of blood flow impinges on the vessel wall. Mizutani et al. classified cerebral dissecting aneurysms into four types. According to their classification, BBAs may be type 4 dissections. Type 4 dissections have focal defects of the internal elastic lamina covered by a thin layer of fibrous tissue and adventitia, and lack the usual collagenous layer, a condition similar to a BBA [17]. Therefore, treatment should not be focused on the BBA sac alone, but also on the affected wall of the ICA itself. Thus, as in a ruptured vertebral artery dissecting aneurysm, ICA trapping seems to be the most favorable treatment method. However, ICA trapping should not be indicated for all patients with BBAs. Depending on the case, the insufficient collateral flow and the originating sites of the essential branches, such as PcomA and AchoA, may make ICA trapping not feasible [19].
Clinical aspects of saccular embolization for BBAs
Though BBA is usually a tiny and broad-necked aneurysm, it often shows rapid configurational changes and occasionally demonstrates a relatively saccular-like shape; a pseudoaneurysm sac often assumes a saccular configuration [13]. The authors treated these saccular-shaped BBAs endovascularly with coil packing of the saccular cavity. This strategy can preserve the ICA flow, though the risks of regrowth and rebleeding remain.
Our series demonstrated satisfactory clinical results and acceptable complication rates, with seven out of nine patients (77.8%), with BBA showing good outcomes (GOS scores, 1–2). Though embolization itself cannot dispose off the lesion completely, we consider that it may temporarily reduce the lesional activity. Consequently, that effect helps the affected ICA wall to heal spontaneously over a period of several weeks or months [16].
Actually, in some cases, the occlusion of a BBA initially achieved by saccular embolization can prevent recurrence and rebleeding for a long period. In other cases, embolization may also temporarily prevent recurrence and rebleeding, though it may be insufficient to prevent BBA regrowth/rebleeding. Thus, a very short-term angiographic follow-up is mandatory. In our series, two of seven patients (28.6%) who had a regrowth of their aneurysm underwent an additional coil embolization, uneventfully. Subsequently, both recurrent BBAs have been stable. Fortunately, rebleeding was not observed in our series.
As for the treatment time, it might be better to wait until the subacute or chronic phase when the wall of the lesion becomes more stable and sometimes progresses to a more saccular appearance for coil embolization to be successful, while not overlooking the risk of aneurysm rerupture.
Recently, there have been some reports on the endosaccular embolization of BBA [1–4, 13–15, 19, 23]. Table 2 shows a summary of BBAs treated by saccular coil embolization previously described in the literature (initial stent-assisted coil embolization cases are excluded). Among those 22 cases, 15 (68.2%) saccular coil embolizations were not followed by additional treatment. Intraoperative ruptures occurred in only two (9.1%). Although the recurrence/regrowth rate was high (38.1%), in 14 of 22 patients (63.6%), a good recovery was confirmed. As for the treatment time, the acute period and subacute/chronic period were 9 (40.9%) and 13 (59.1%) of 22 patients, respectively. These results were similar to the data obtained in our series.
Technical aspect of saccular embolization for BBAs
In fact, BBAs are difficult to treat with coil embolization, owing to their small size, wide neck, and location, making it technically difficult to place coils in the cavity [15]. Appropriate steam-shaping of the microcatheter tip and a balloon-assisted technique may help to overcome the difficulty of coiling the BBA. An appropriately-shaped microcatheter tip and positioning of the balloon in ICA help to prevent catheter kickback during embolization. Moreover, a balloon positioned across the BBA may be used to dam the flow of the ICA if a BBA were to rupture during the procedure. Among our cases, only one intraprocedural rupture occurred, and balloon inflation then proved very useful to control the bleeding (case 6).
Stent-assisted coil embolization may be a useful and considerable option for stabilizing the inserted coils inside such a tiny aneurysm [9, 10, 12, 19]. However, the presence of a stent makes microcatheter manipulation difficult, and perioperative anticoagulate/antiplatelet therapy is mandatory for intracranial stenting. Moreover, from a technical standpoint, stent deployment within the affected ICA, potentially involves the risk of vessel rupture.
In their recently published paper, Hong and colleagues presented a variation of the semi-jailing, stent-assisted technique as applied to blister-type aneurysms [5]. Coils are first placed in the vicinity of the aneurysm neck, and the stent is subsequently deployed to constrain the coils within the aneurysm. This technique seems to be a new treatment option, but the technical difficulty and potential risk related to stenting must be further investigated.
Alternative treatment
Various surgical strategies have been reported, including parallel clip placement, wrapping, clip-on wrapping, and ICA trapping with, or without a bypass. Regardless of the surgical modality, direct surgical approaches pose a high risk of premature intraoperative rupture and ICA laceration [15, 18, 21, 22]. As was shown in one of our cases (case 7), wrapping of the aneurysm does not seem to prevent regrowth.
Recently, an extracranial–intracranial (EC–IC) high-flow bypass, followed by trapping of the aneurysm occluding the ICA, has been reported [2, 6, 8]. This strategy may achieve complete aneurysm occlusion, thus, preventing severe postoperative cerebral ischemia due to ICA occlusion. However, preparing a high-flow bypass for SAH patients still poses a technical difficulty, while the direct approach to ICA results in a higher incidence of premature rupture.
Various endovascular strategies have also been documented, including endovascular trapping [19], overlap stenting, and flow-diverter stenting [9, 11, 12]. Endovascular ICA trapping is an optimal treatment for patients' tolerance to ICA obstruction. However, it is difficult to precisely evaluate the result of balloon occlusion tests for SAH patients, as shown in one of our cases (case 8). Furthermore, as was already mentioned, endovascular trapping is unsuitable when PcomA or AchoA originates too close to the lesion. Although an EC–IC bypass and endovascular trapping also constitute one treatment option, the risk of thromboembolic or hemorrhagic complications must also be considered in this combined therapy.
Overlap stenting or flow-diverter stenting can preserve the flow of a parent vessel and may overcome the drawbacks of saccular embolization [9, 11, 12]. However, the potential risk of regrowth/rebleeding in relation to perioperative anticoagulate/antiplatelet therapy remains. Recurrent SAHs during anticoagulate/antiplatelet therapy may prove fatal for the patient. Furthermore, as already mentioned, stent deployment within the affected ICA runs the potential risk of vessel rupture. However, it is expected that the development of new stent technology that promotes vascular neointima formation and generates less platelet activation and aggregation might suffice to overcome such problems.
Conclusions
Endovascular coil embolization can be considered as an alternative treatment option for the saccular-shaped BBAs in selected patients for whom ICA sacrifice is not feasible.
Abbreviations
- AchoA:
-
anterior choroidal artery
- BBA:
-
blood blister-like aneurysm
- CT:
-
computed tomography
- GOS:
-
Glasgow Outcome Scale
- ICA:
-
internal carotid artery
- MRA:
-
MR angiogram
- PcomA:
-
posterior communicating artery
- SAH:
-
subarachnoid hemorrhage
References
Ahn JY, Kwon SO, Joo JY (2001) Dorsal internal carotid artery aneurysm treated by coil embolization—case report. Neurol Med Chir (Tokyo) 41:603–605
Başkaya MK, Ahmed AS, Ateş O, Niemann D (2008) Surgical treatment of blood blister-like aneurysms of the supraclinoid internal carotid artery with extracranial-intracranial bypass and trapping. Neurosurg Focus 24:E13
Doorenbosch X, Harding M (2008) Primary treatment of a blood-blister-like aneurysm of the internal carotid artery with Guglielmi detachable coil embolisation. J Clin Neurosci 15:1276–1279
Ezaki Y, Takahata H, Kamada K, Baba S, Kaminogo M (2006) Aneurysmal embolization of a blisterlike aneurysm of the internal carotid artery: a case report and review of the literature. Surg Neurol 65:628–630
Hong B, Patel NV, Gounis MJ, DeLeo MJ 3rd, Linfante I, Wojak JC, Wakhloo AK (2009) Semi-jailing technique for coil embolization of complex, wide-necked intracranial aneurysms. Neurosurgery 65:1131–1138
Ishikawa T, Mutoh T, Nakayama N, Yasuda H, Nomura M, Kazumata K, Moroi J, Yasui N (2009) Universal external carotid artery to proximal middle cerebral artery bypass with interposed radial artery graft prior to approaching ruptured blood blister-like aneurysm of the internal carotid artery. Neurol Med Chir (Tokyo) 49:553–558
Ishikawa T, Nakamura N, Houkin K, Nomura M (1997) Pathological consideration of a “blister-like” aneurysm at the superior wall of the internal carotid artery: case report. Neurosurgery 40:403–405
Kamijo K, Matsui T (2010) Acute extracranial-intracranial bypass using a radial artery graft along with trapping of a ruptured blood blister–like aneurysm of the internal carotid artery. Clinical artery. J Neurosurg 113:781–785. doi:10.3171/2009.10.JNS09970
Kim BM, Chung EC, Park SI, Choi CS, Won YS (2007) Treatment of blood blister-like aneurysm of the internal carotid artery with stent-assisted coil embolization followed by stent-within-a-stent technique. Case report. J Neurosurg 107:1211–1213
Korja M, Rautio R, Valtonen S, Haapanen A (2008) Primary treatment of ruptured blood blister-like aneurysms with stent-assisted coil embolization: report of two cases. Acta Radiol 49:180–183
Kulcsár Z, Wetzel SG, Augsburger L, Gruber A, Wanke I, Rüfenacht DA (2010) Effect of flow diversion treatment on very small ruptured aneurysms. Neurosurgery 67:789–793
Lee BH, Kim BM, Park MS, Park SI, Chung EC, Suh SH, Choi CS, Won YS, Yu IK (2009) Reconstructive endovascular treatment of ruptured blood blister-like aneurysms of the internal carotid artery. J Neurosurg 110:431–436
Lee CC, Hsieh TC, Wang YC, Lo YL, Lee ST, Yang TC (2010) Ruptured symptomatic internal carotid artery dorsal wall aneurysm with rapid configurational change: clinical experience and management outcome. Eur J Neurol 17:1277–1284. doi:10.1111/j.1468-1331.2010.03029.x
McNeely PD, Clarke DB, Baxter B, Vandorpe RA, Mendez I (2002) Endovascular treatment of a “blister-like” aneurysm on the internal carotid artery. Can J Neurol Sci 27:247–250
Meling TR, Sorteberg A, Bakke SJ, Slettebo H, Hernesniemi J, Sorteberg W (2008) Blood blister-like aneurysms of the internal carotid artery trunk causing subarachnoid hemorrhage: treatment and outcome. J Neurosurg 108:662–671
Mizutani T, Kojima H, Asamoto S (2004) Healing process for cerebral dissecting aneurysms presenting with subarachnoid hemorrhage. Neurosurgery 54:342–347
Mizutani T, Miki Y, Kojima H, Suzuki H (1999) Proposed classification of nonatherosclerotic cerebral fusiform and dissecting aneurysms. Neurosurgery 45:253–259
Ogawa A, Suzuki M, Ogasawara K (2000) Aneurysms at nonbranching sites in the surpaclinoid portion of the internal carotid artery: internal carotid artery trunk aneurysms. Neurosurgery 47:578–583
Park JH, Park IS, Han DH, Kim SH, Oh CW, Kim JE, Kim JH, Han MH, Kwon OK (2007) Endovascular treatment of blood blister-like aneurysms of the internal carotid artery. J Neurosurg 106:812–819
Sano K (1996) Concerning the nomenclature and classification of internal carotid aneurysms. Surg Cereb Stroke 24:333–339, Jpn
Satoh A, Sugiyama T, Hongo K, Kakizawa Y, Ishihara S, Matsutani M (2008) Nationwide surveillance of IC anterior (or dorsal) wall aneurysm: with special reference to its dissecting nature. Acta Neurochir Suppl 103:51–55
Sim SY, Shin YS, Cho KG, Kim SY, Kim SH, Ahn YH, Yoon SH, Cho KH (2006) Blood blister-like aneurysms at nonbranching sites of the internal carotid artery. J Neurosurg 105:400–405
Tanoue S, Kiyosue H, Matsumoto S, Yamashita M, Nagatomi H, Mori H (2004) Ruptured “blisterlike” aneurysm with a pseudoaneurysm formation requiring delayed intervention with endovascular coil embolization. Case report. J Neurosurg 101:159–162
Acknowledgments
The authors wish to thank Takeshi Okamoto, M.D. of the Japanese Red Cross, Nagoya Daiichi Hospital, and Takeshi Kinkori, M.D. of the Okazaki Municipal Hospital for their generous support during this study.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matsubara, N., Miyachi, S., Tsukamoto, N. et al. Endovascular coil embolization for saccular-shaped blood blister-like aneurysms of the internal carotid artery. Acta Neurochir 153, 287–294 (2011). https://doi.org/10.1007/s00701-010-0898-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-010-0898-9