Abstract
While it has long been thought that most of cerebral creatine is of peripheral origin, the last 20 years has provided evidence that the creatine synthetic pathway (AGAT and GAMT enzymes) is expressed in the brain together with the creatine transporter (SLC6A8). It has also been shown that SLC6A8 is expressed by microcapillary endothelial cells at the blood–brain barrier, but is absent from surrounding astrocytes, raising the concept that the blood–brain barrier has a limited permeability for peripheral creatine. The first creatine deficiency syndrome in humans was also discovered 20 years ago (GAMT deficiency), followed later by AGAT and SLC6A8 deficiencies, all three diseases being characterized by creatine deficiency in the CNS and essentially affecting the brain. By reviewing the numerous and latest experimental studies addressing creatine transport and synthesis in the CNS, as well as the clinical and biochemical characteristics of creatine-deficient patients, our aim was to delineate a clearer view of the roles of the blood–brain and blood-cerebrospinal fluid barriers in the transport of creatine and guanidinoacetate between periphery and CNS, and on the intracerebral synthesis and transport of creatine. This review also addresses the question of guanidinoacetate toxicity for brain cells, as probably found under GAMT deficiency.




Similar content being viewed by others
Abbreviations
- 3D:
-
Three-dimensional
- AChE:
-
Acetylcholine esterase
- AGAT:
-
Arginine, glycine amidinotransferase
- AMPK:
-
AMP-activated protein kinase
- Arg:
-
Arginine
- BBB:
-
Blood–brain barrier
- BCSFB:
-
Blood–CSF barrier
- CCDS:
-
Cerebral creatine deficiency syndromes
- CK:
-
Creatine kinase
- CNS:
-
Central nervous system
- Cr:
-
Creatine
- Crn:
-
Creatinine
- CSF:
-
Cerebrospinal fluid
- GAA:
-
Guanidinoacetate
- GABA:
-
γ-Amino butyric acid
- GABAA-R and GABAB-R:
-
GABAA and GABAB receptors
- GAD:
-
Glutamic acid decarboxylase
- GAMT:
-
Guanidinoacetate methyltransferase
- Gly:
-
Glycine
- ID/DD:
-
Intellectual/development delays
- MCEC:
-
Microcapillary endothelial cells
- MCT12:
-
Monocarboxylate transporter 12
- MRS:
-
Magnetic resonance spectroscopy
- Orn:
-
Ornithine
- PCr:
-
Phosphocreatine
- PGAA:
-
Phosphorylated GAA
- SGK:
-
Serum/glucocorticoid-regulated kinase
- SLC6A8:
-
Creatine transporter
- TauT:
-
Taurine transporter
References
Abplanalp J, Laczko E, Philp N et al (2013) The cataract and glucosuria associated monocarboxylate transporter MCT12 is a new creatine transporter. Hum Mol Genet 22:3218–3226
Adriano E, Garbati P, Damonte G, Salis A, Armirotti A, Balestrino M (2011) Searching for a therapy of creatine transporter deficiency: some effects of creatine ethyl ester in brain slices in vitro. Neuroscience 199:386–393
Alfieri RR, Bonelli MA, Cavazzoni et al (2006) Creatine as a compatible osmolyte in muscle cells exposed to hypertonic stress. J Physiol 576:391–401
Almeida LS, Salomons GS, Hogenboom F, Jakobs C, Schoffelmeer AN (2006) Exocytotic release of creatine in rat brain. Synapse 60(118):123
Arias A, Corbella M, Fons C et al (2007) Creatine transporter deficiency: prevalence among patients with mental retardation and pitfalls in metabolite screening. Clin Biochem 40:1328–1331
Baroncelli L, Alessandrì M, Tola J et al (2014) A novel mouse model of creatine transporter deficiency. F1000Res 3:228
Battini R, Leuzzi V, Carducci C et al (2002) Creatine depletion in a new case with AGAT deficiency: clinical and genetic study in a large pedigree. Mol Genet Metab 77:326–331
Battini R, Alessandri MG, Leuzzi V, Moro F, Tosetti M, Bianchi MC, Cioni G (2006) Arginine:glycine amidinotransferase (AGAT) deficiency in a newborn: early treatment can prevent phenotypic expression of the disease. J Pediatr 148:828–830
Beal MF (2011) Neuroprotective effects of creatine. Amino Acids 40:1305–1313
Béard E, Braissant O (2010) Synthesis and transport of creatine in the CNS: importance for cerebral functions. J Neurochem 115:297–313
Bianchi MC, Tosetti M, Fornai F, Alessandri MG, Cipriani P, De Vito G, Canapicchi R (2000) Reversible brain creatine deficiency in two sisters with normal blood creatine level. Ann Neurol 47:511–513
Bizzi A, Bugiani M, Salomons GS et al (2002) X-linked creatine deficiency syndrome: a novel mutation in creatine transporter gene SLC6A8. Ann Neurol 52:227–231
Bothwell JH, Rae C, Dixon RM, Styles P, Bhakoo KK (2001) Hypo-osmotic swelling-activated release of organic osmolytes in brain slices: implications for brain oedema in vivo. J Neurochem 77:1632–1640
Bothwell JH, Styles P, Bhakoo KK (2002) Swelling-activated taurine and creatine effluxes from rat cortical astrocytes are pharmacologically distinct. J Membr Biol 185:157–164
Brady ST, Lasek RJ (1981) Nerve-specific enolase and creatine phosphokinase in axonal transport: soluble proteins and the axoplasmic matrix. Cell 23:515–523
Braissant O (2012) Creatine and guanidinoacetate transport at blood-brain and blood-cerebrospinal fluid barriers. J Inherit Metab Dis 35:655–664
Braissant O (2014) GAMT deficiency: 20 years of a treatable inborn error of metabolism. Mol Genet Metab 111:1–3
Braissant O, Henry H (2008) AGAT, GAMT and SLC6A8 distribution in the central nervous system, in relation to creatine deficiency syndromes: a review. J Inherit Metab Dis 31:230–239
Braissant O, Henry H, Loup M, Eilers B, Bachmann C (2001) Endogenous synthesis and transport of creatine in the rat brain: an in situ hybridization study. Mol Brain Res 86:193–201
Braissant O, Henry H, Villard AM et al (2002) Ammonium-induced impairment of axonal growth is prevented through glial creatine. J Neurosci 22:9810–9820
Braissant O, Henry H, Villard AM, Speer O, Wallimann T, Bachmann C (2005) Creatine synthesis and transport during rat embryogenesis: spatiotemporal expression of AGAT, GAMT and CT1. BMC Dev Biol 5:9
Braissant O, Cagnon L, Monnet-Tschudi F, Speer O, Wallimann T, Honegger P, Henry H (2008) Ammonium alters creatine transport and synthesis in a 3D-culture of developing brain cells, resulting in secondary cerebral creatine deficiency. Eur J Neurosci 27:1673–1685
Braissant O, Béard E, Torrent C, Henry H (2010) Dissociation of AGAT, GAMT and SLC6A8 in CNS: relevance to creatine deficiency syndromes. Neurobiol Dis 37:423–433
Braissant O, Henry H, Beard E, Uldry J (2011) Creatine deficiency syndromes and the importance of creatine synthesis in the brain. Amino Acids 40:1315–1324
Braissant O, McLin VA, Cudalbu C (2013) Ammonia toxicity to the brain. J Inherit Metab Dis 36:595–612
Brosnan JT, Brosnan ME (2007) Creatine: endogenous metabolite, dietary, and therapeutic supplement. Annu Rev Nutr 27:241–261
Brosnan ME, Edison EE, da Silva R, Brosnan JT (2007) New insights into creatine function and synthesis. Adv Enzyme Regul 47:252–260
Brosnan JT, Wijekoon EP, Warford-Woolgar L, Trottier NL, Brosnan ME, Brunton JA, Bertolo RF (2009) Creatine synthesis is a major metabolic process in neonatal piglets and has important implications for amino acid metabolism and methyl balance. J Nutr 139:1292–1297
Brosnan JT, da Silva RP, Brosnan ME (2011) The metabolic burden of creatine synthesis. Amino Acids 40:1325–1331
Burov S, Leko M, Dorosh M, Dobrodumov A, Veselkina O (2011) Creatinyl amino acids-new hybrid compounds with neuroprotective activity. J Pept Sci 17:620–626
Cecil KM, Salomons GS, Ball WS et al (2001) Irreversible brain creatine deficiency with elevated serum and urine creatine: a creatine transporter defect? Ann Neurol 49:401–404
Ceddia RB, Sweeney G (2004) Creatine supplementation increases glucose oxidation and AMPK phosphorylation and reduces lactate production in L6 rat skeletal muscle cells. J Physiol 555:409–421
Chen NH, Reith ME, Quick MW (2004) Synaptic uptake and beyond: the sodium- and chloride-dependent neurotransmitter transporter family SLC6. Pflugers Arch 447:519–531
Chilosi A, Leuzzi V, Battini R et al (2008) Treatment with l-arginine improves neuropsychological disorders in a child with creatine transporter defect. Neurocase 14:151–161
Choe CU, Atzler D, Wild P et al (2013a) Homoarginine levels are regulated by l-arginine:glycine amidinotransferase and affect stroke outcome: results from human and murine studies. Circulation 128:1451–1461
Choe CU, Nabuurs C, Stockebrand MC et al (2013b) l-arginine:glycine amidinotransferase deficiency protects from metabolic syndrome. Hum Mol Genet 22:110–123
Clark AJ, Rosenberg EH, Almeida LS et al (2006) X-linked creatine transporter (SLC6A8) mutations in about 1% of males with mental retardation of unknown etiology. Hum Genet 119:604–610
Craigen WJ, Stromberger C, Donti T, Raghavan A, Ather S, Wehrens X, Akman HO (2011) A mouse model of creatine deficiency due to targeted disruption of Alanine: glycine Amidinotransferase exhibits muscle hypoplasia, mitochondrial dysfunction, and weakness. J Inherit Metab Dis 34:125
Cunha M, Martín-de-Saavedra M, Romero A et al (2014) Both creatine and its product phosphocreatine reduce oxidative stress and afford neuroprotection in an in vitro Parkinson’s model. ASN Neuro 24:6
da Silva RP, Nissim I, Brosnan ME, Brosnan JT (2009) Creatine synthesis: hepatic metabolism of guanidinoacetate and creatine in the rat in vitro and in vivo. Am J Physiol Endocrinol Metab 296:E256–E261
Darrabie M, Arciniegas A, Mishra R, Bowles D, Jacobs D, Santacruz L (2011) AMPK and substrate availability regulate creatine transport in cultured cardiomyocytes. Am J Physiol Endocrinol Metab 300:E870–E876
Davis BM, Miller RK, Brent RL, Koszalka TR (1978) Materno-fetal transport of creatine in the rat. Biol Neonate 33:43–54
De Deyn PP, Marescau B, Macdonald RL (1991) Guanidino compounds that are increased in hyperargininemia inhibit GABA and glycine responses on mouse neurons in cell culture. Epilepsy Res 8:134–141
DeGrauw TJ, Salomons GS, Cecil KM, Chuck G, Newmeyer A, Schapiro MB, Jakobs C (2002) Congenital creatine transporter deficiency. Neuropediatrics 33:232–238
Dolder M, Walzel B, Speer O, Schlattner U, Wallimann T (2003) Inhibition of the mitochondrial permeability transition by creatine kinase substrates. Requirement for microcompartmentation. J Biol Chem 278:17760–17766
Ensenauer R, Thiel T, Schwab KO et al (2004) Guanidinoacetate methyltransferase deficiency: differences of creatine uptake in human brain and muscle. Mol Genet Metab 82:208–213
Fitch CD, Shields RP, Payne WF, Dacus JM (1968) Creatine metabolism in skeletal muscle. 3. Specificity of the creatine entry process. J Biol Chem 243:2024–2027
Fons C, Sempere A, Arias A et al (2008) Arginine supplementation in four patients with X-linked creatine transporter defect. J Inherit Metab Dis 31:724–728
Galbraith RA, Furukawa M, Li M (2006) Possible role of creatine concentrations in the brain in regulating appetite and weight. Brain Res 1101:85–91
Ganesan V, Johnson A, Connelly A, Eckhardt S, Surtees RA (1997) Guanidinoacetate methyltransferase deficiency: new clinical features. Pediatr Neurol 17:155–157
Guthmiller P, Van Pilsum JF, Boen JR, McGuire DM (1994) Cloning and sequencing of rat kidney l-arginine:glycine amidinotransferase. Studies on the mechanism of regulation by growth hormone and creatine. J Biol Chem 269:17556–17560
Hanna-El-Daher L, Béard E, Braissant O, Henry H, Tenenbaum L (2015) Mild guanidinoacetate increase under partial GAMT deficiency strongly affects brain cell development. Neurobiol Dis 79:14–27
Hautman E, Kokenge A, Udobi K, Williams M, Vorhees C, Skelton M (2014) Female mice heterozygous for creatine transporter deficiency show moderate cognitive deficits. J Inherit Metab Dis 37:63–68
Ireland Z, Dickinson H, Snow R, Walker DW (2008) Maternal creatine: does it reach the fetus and improve survival after an acute hypoxic episode in the spiny mouse (Acomys cahirinus)? Am J Obstet Gynecol 198:431–436
Ireland Z, Russell AP, Wallimann T, Walker DW, Snow R (2009) Developmental changes in the expression of creatine synthesizing enzymes and creatine transporter in a precocial rodent, the spiny mouse. BMC Dev Biol 9:39
Item CB, Stöckler-Ipsiroglu S, Stromberger C et al (2001) Arginine:glycine amidinotransferase deficiency: the third inborn error of creatine metabolism in humans. Am J Hum Genet 69:1127–1133
Kaldis P, Hemmer W, Zanolla E, Holtzman D, Wallimann T (1996) ‘Hot spots’ of creatine kinase localization in brain: cerebellum, hippocampus and choroid plexus. Dev Neurosci 18:542–554
Kan HE, Meeuwissen E, van Asten JJ, Veltien A, Isbrandt D, Heerschap A (2007) Creatine uptake in brain and skeletal muscle of mice lacking guanidinoacetate methyltransferase assessed by magnetic resonance spectroscopy. J Appl Physiol 102:2121–2127
Koga Y, Takahashi H, Oikawa D, Tachibana T, Denbow DM, Furuse M (2005) Brain creatine functions to attenuate acute stress responses through GABAnergic system in chicks. Neuroscience 132:65–71
Kola B (2008) Role of AMP-activated protein kinase in the control of appetite. J Neuroendocrinol 20:942–951
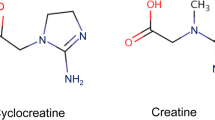
Kurosawa Y, Degrauw TJ, Lindquist DM et al (2012) Cyclocreatine treatment improves cognition in mice with creatine transporter deficiency. J Clin Invest 122:2837–2846
Lee H, Ogawa H, Fujioka M, Gerton GL (1994) Guanidinoacetate methyltransferase in the mouse: extensive expression in Sertoli cells of testis and in microvilli of caput epididymis. Biol Reprod 50:152–162
Lensman M, Korzhevskii DE, Mourovets VO et al (2006) Intracerebroventricular administration of creatine protects against damage by global cerebral ischemia in rat. Brain Res 1114:187–194
Lion-François L, Cheillan D, Pitelet G et al (2006) High frequency of creatine deficiency syndromes in patients with unexplained mental retardation. Neurology 67:1713–1714
Lowe MT, Faull RL, Christie DL, Waldvogel HJ (2015) Distribution of the creatine transporter throughout the human brain reveals a spectrum of creatine transporter immunoreactivity. J Comp Neurol 523:699–725
Mak CS, Waldvogel HJ, Dodd JR et al (2009) Immunohistochemical localisation of the creatine transporter in the rat brain. Neuroscience 163:571–585
Mercimek-Mahmutoglu S, Stöckler-Ipsiroglu S, Adami A et al (2006) GAMT deficiency: features, treatment, and outcome in an inborn error of creatine synthesis. Neurology 67:480–484
Mercimek-Mahmutoglu S, Ndika J, Kanhai W et al (2014) Thirteen new patients with guanidinoacetate methyltransferase deficiency and functional characterization of nineteen novel missense variants in the GAMT gene. Human Mut 35:462–469
Möller A, Hamprecht B (1989) Creatine transport in cultured cells of rat and mouse brain. J Neurochem 52:544–550
Nabuurs CI, Choe CU, Veltien A et al (2013) Disturbed energy metabolism and muscular dystrophy caused by pure creatine deficiency are reversible by creatine intake. J Physiol 591:571–592
Neu A, Neuhoff H, Trube G, Fehr S, Ullrich K, Roeper J, Isbrandt D (2002) Activation of GABA(A) receptors by guanidinoacetate: a novel pathophysiological mechanism. Neurobiol Dis 11:298–307
O’Gorman E, Beutner G, Dolder M, Koretsky AP, Brdiczka D, Wallimann T (1997) The role of creatine kinase in inhibition of mitochondrial permeability transition. FEBS Let 414:253–257
Ohtsuki S, Tachikawa M, Takanaga H, Shimizu H, Watanabe M, Hosoya K, Terasaki T (2002) The blood-brain barrier creatine transporter is a major pathway for supplying creatine to the brain. J Cereb Blood Flow Metab 22:1327–1335
Peral MJ, Vazquez-Carretero MD, Ilundain AA (2010) Na(+)/Cl(−)/creatine transporter activity and expression in rat brain synaptosomes. Neuroscience 165:53–60
Perasso L, Cupello A, Lunardi GL, Principato C, Gandolfo C, Balestrino M (2003) Kinetics of creatine in blood and brain after intraperitoneal injection in the rat. Brain Res 974:37–42
Perasso L, Lunardi GL, Risso F et al (2008) Protective effects of some creatine derivatives in brain tissue anoxia. Neurochem Res 33:765–775
Perasso L, Adriano E, Ruggeri P, Burov SV, Gandolfo C, Balestrino M (2009) In vivo neuroprotection by a creatine-derived compound: phosphocreatine-Mg-complex acetate. Brain Res 1285:158–163
Póo-Argüelles P, Arias A, Vilaseca MA et al (2006) X-Linked creatine transporter deficiency in two patients with severe mental retardation and autism. J Inherit Metab Dis 29:220–223
Renema WK, Schmidt A, van Asten JJ et al (2003) MR spectroscopy of muscle and brain in guanidinoacetate methyltransferase (GAMT)-deficient mice: validation of an animal model to study creatine deficiency. Magn Reson Med 50:936–943
Rosenberg EH, Almeida LS, Kleefstra T et al (2004) High prevalence of SLC6A8 deficiency in X-linked mental retardation. Am J Hum Genet 75:97–105
Salomons GS, van Dooren SJ, Verhoeven NM, Cecil KM, Ball WS, DeGrauw TJ, Jakobs C (2001) X-linked creatine-transporter gene (SLC6A8) defect: a new creatine-deficiency syndrome. Am J Hum Genet 68:1497–1500
Sandell LL, Guan XJ, Ingram R, Tilghman SM (2003) Gatm, a creatine synthesis enzyme, is imprinted in mouse placenta. Proc Natl Acad Sci USA 100:4622–4627
Schlattner U, Tokarska-Schlattner M, Wallimann T (2006) Mitochondrial creatine kinase in human health and disease. Biochim Biophys Acta 1762:164–180
Schloss P, Mayser W, Betz H (1994) The putative rat choline transporter CHOT1 transports creatine and is highly expressed in neural and muscle-rich tissues. Biochem Biophys Res Commun 198:637–645
Schmidt A, Marescau B, Boehm EA et al (2004) Severely altered guanidino compound levels, disturbed body weight homeostasis and impaired fertility in a mouse model of guanidinoacetate N-methyltransferase (GAMT) deficiency. Hum Mol Genet 13:905–921
Schulze A, Battini R (2007) Pre-symptomatic treatment of creatine biosynthesis defects. Subcell Biochem 46:167–181
Schulze A, Hess T, Wevers R et al (1997) Creatine deficiency syndrome caused by guanidinoacetate methyltransferase deficiency: diagnostic tools for a new inborn error of metabolism. J Pediatr 131:626–631
Schulze A, Ebinger F, Rating D, Mayatepek E (2001) Improving treatment of guanidinoacetate methyltransferase deficiency: reduction of guanidinoacetic acid in body fluids by arginine restriction and ornithine supplementation. Mol Genet Metab 74:413–419
Schulze A, Hoffmann GF, Bachert P, Kirsch S, Salomons GS, Verhoeven NM, Mayatepek E (2006) Presymptomatic treatment of neonatal guanidinoacetate methyltransferase deficiency. Neurology 67:719–721
Sestili P, Martinelli C, Bravi G et al (2006) Creatine supplementation affords cytoprotection in oxidatively injured cultured mammalian cells via direct antioxidant activity. Free Radic Biol Med 40:837–849
Shiraga H, Watanabe Y, Mori A (1991) Guanidino compound levels in the serum of healthy adults and epileptic patients. Epilepsy Res 8:142–148
Shojaiefard M, Christie DL, Lang F (2005) Stimulation of the creatine transporter SLC6A8 by the protein kinases SGK1 and SGK3. Biochem Biophys Res Commun 334:742–746
Shojaiefard M, Christie DL, Lang F (2006) Stimulation of the creatine transporter SLC6A8 by the protein kinase mTOR. Biochem Biophys Res Commun 341:945–949
Sijens PE, Verbruggen KT, Oudkerk M, van Spronsen FJ, Soorani-Lunsing RJ (2005) 1H MR spectroscopy of the brain in Cr transporter defect. Mol Genet Metab 86:421–422
Sinha A, Ahmed S, von Both I, Isbrandt D, Henkelman R, Schulze A (2011) Anatomical phenotyping in mouse models of AGAT and GAMT deficiency with magnetic resonance imaging. J Inherit Metab Dis 34:124
Skelton MR, Schaefer TL, Graham DL, DeGrauw TJ, Clark JF, Williams MT, Vorhees CV (2011) Creatine transporter (CrT; Slc6a8) knockout mice as a model of human CrT deficiency. PLoS One 6:e16187
Stöckler S, Holzbach U, Hanefeld F et al (1994) Creatine deficiency in the brain: a new, treatable inborn error of metabolism. Pediatr Res 36:409–413
Stöckler S, Hanefeld F, Frahm J (1996a) Creatine replacement therapy in guanidinoacetate methyltransferase deficiency, a novel inborn error of metabolism. Lancet 348:789–790
Stöckler S, Isbrandt D, Hanefeld F, Schmidt B, Von Figura K (1996b) Guanidinoacetate methyltransferase deficiency: the first inborn error of creatine metabolism in man. Am J Hum Genet 58:914–922
Stöckler S, Braissant O, Schulze A (2014) Creatine disorders. Physician’s guide to the diagnosis, treatment, and follow-up of inherited metabolic diseases (Berlin, Heidelberg. Springer-Verlag), New-York, pp 529–540
Stöckler-Ipsiroglu S, van Karnebeek C (2014) Cerebral creatine deficiencies: a group of treatable intellectual developmental disorders. Semin Neurol 34:350–356
Stöckler-Ipsiroglu S, van Karnebeek C, Longo N et al (2014) Guanidinoacetate methyltransferase (GAMT) deficiency: outcomes in 48 individuals and recommendations for diagnosis, treatment and monitoring. Mol Genet Metab 111:16–25
Stöckler-Ipsiroglu S, Apatean D, Battini R et al (2015) Arginine:glycine amidinotransferase (AGAT) deficiency: Clinical features and long term outcomes in 16 patients diagnosed worldwide. Mol Genet Metab (in press)
Strutz-Seebohm N, Shojaiefard M, Christie D, Tavare J, Seebohm G, Lang F (2007) PIKfyve in the SGK1 mediated regulation of the creatine transporter SLC6A8. Cell Physiol Biochem 20:729–734
Tachikawa M, Fukaya M, Terasaki T, Ohtsuki S, Watanabe M (2004) Distinct cellular expressions of creatine synthetic enzyme GAMT and creatine kinases uCK-Mi and CK-B suggest a novel neuron-glial relationship for brain energy homeostasis. Eur J Neurosci 20:144–160
Tachikawa M, Fujinawa J, Takahashi M et al (2008) Expression and possible role of creatine transporter in the brain and at the blood-cerebrospinal fluid barrier as a transporting protein of guanidinoacetate, an endogenous convulsant. J Neurochem 107:768–778
Tachikawa M, Kasai Y, Yokoyama R, Fujinawa J, Ganapathy V, Terasaki T, Hosoya KI (2009) The blood-brain barrier transport and cerebral distribution of guanidinoacetate in rats: involvement of creatine and taurine transporters. J Neurochem 111:499–509
Takeda M, Kiyatake I, Koide H, Jung KY, Endou H (1992) Biosynthesis of guanidinoacetic acid in isolated renal tubules. Eur J Clin Chem Clin Biochem 30:325–331
Torremans A, Marescau B, Possemiers I, Van Dam D, D’Hooge R, Isbrandt D, De Deyn PP (2005) Biochemical and behavioural phenotyping of a mouse model for GAMT deficiency. J Neurol Sci 231:49–55
Trotier-Faurion A, Dézard S, Taran F, Valayannopoulos V, de Lonlay P, Mabondzo A (2013) Synthesis and biological evaluation of new creatine fatty esters revealed dodecyl creatine ester as a promising drug candidate for the treatment of the creatine transporter deficiency. J Med Chem 56:5173–5181
Trotier-Faurion A, Passirani C, Béjaud J et al (2015) Dodecyl creatine ester and lipid nanocapsule: a double strategy for the treatment of creatine transporter deficiency. Nanomedicine 10:185–191
Valayannopoulos V, Boddaert N, Chabli A et al (2012) Treatment by oral creatine, l-arginine and l-glycine in six severely affected patients with creatine transporter defect. J Inherit Metab Dis 35:151–157
van de Kamp JM, Mancini GM, Pouwels PJ et al (2011) Clinical features and X-inactivation in females heterozygous for creatine transporter defect. Clin Genet 79:264–272
van de Kamp JM, Jakobs C, Gibson KM, Salomons GS (2013) New insights into creatine transporter deficiency: the importance of recycling creatine in the brain. J Inherit Metab Dis 36:155–156
Vlasov T, Chefu S, Baĭsa A, Leko M, Burov S, Veselkina O (2011) Amides of creatine: perspectives of neuroprotection. Ross Fiziol Zh Im I M Sechenova 97:708–717
Walker JB, Hannan JK (1976) Creatine biosynthesis during embryonic development. False feedback suppression of liver amidinotransferase by N-acetimidoylsarcosine and 1-carboxymethyl-2-iminoimidazolidine (cyclocreatine). Biochemistry 15:2519–2522
Wallimann T, Hemmer W (1994) Creatine kinase in non-muscle tissues and cells. Mol Cell Biochem 133–134:193–220
Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger HM (1992) Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem J 281:21–40
Wallimann T, Dolder M, Schlattner U et al (1998) Some new aspects of creatine kinase (CK): compartmentation, structure, function and regulation for cellular and mitochondrial bioenergetics and physiology. BioFactors 8:229–234
Wallimann T, Tokarska-Schlattner M, Neumann D et al (2007) The phosphocreatine circuit: Molecular and cellular physiology of creatine kinases, sensitivity to free radicals and enhancement of creatine supplementation. In: Saks VA (ed) Molecular systems bioenergetics: Energy for life, basic principles, organization and dynamics of cellular energetics. Wiley VCH-Publisher Co., Weinheim, pp 195–264
Wallimann T, Tokarska-Schlattner M, Schlattner U (2011) The creatine kinase system and pleiotropic effects of creatine. Amino Acids 40:1271–1296
Wang L, Zhang Y, Shao M, Zhang H (2007) Spatiotemporal expression of the creatine metabolism related genes agat, gamt and ct1 during zebrafish embryogenesis. Int J Dev Biol 51:247–253
Wilcken B, Fagan E, Sim K, Carpenter KH, Salomons GS (2008) Creatine transporter defect: results of 6 months’ treatment. J Inherit MetabDis 31(Suppl):70
Wyss M, Kaddurah-Daouk R (2000) Creatine and creatinine metabolism. Physiol Rev 80:1107–1213
Zugno AI, Stefanello FM, Streck EL, Calcagnotto T, Wannmacher CM, Wajner M, Wyse AT (2003) Inhibition of Na + , K + -ATPase activity in rat striatum by guanidinoacetate. Int J Dev Neurosci 21:183–189
Zugno AI, Franzon R, Chiarani F, Bavaresco CS, Wannmacher CM, Wajner M, Wyse AT (2004) Evaluation of the mechanism underlying the inhibitory effect of guanidinoacetate on brain Na+, K+-ATPase activity. Int J Dev Neurosci 22:191–196
Zugno AI, Scherer EB, Schuck PF et al (2006) Intrastriatal administration of guanidinoacetate inhibits Na+, K+-ATPase and creatine kinase activities in rat striatum. Metab Brain Dis 21:41–50
Zugno A, Oliveira D, Scherer E, Wajner M, Wofchuk S, Wyse A (2007) Guanidinoacetate inhibits glutamate uptake in rat striatum of rats at different ages. Neurochem Res 32:959–964
Zugno A, Stefanello F, Scherer E et al (2008a) Guanidinoacetate decreases antioxidant defenses and total protein sulfhydryl content in striatum of rats. Neurochem Res 33:1804–1810
Zugno AI, Pereira L, Mattos C, Scherer EB, Netto C, Wyse AT (2008b) Guanidinoacetate administration increases acetylcholinesterase activity in striatum of rats and impairs retention of an inhibitory avoidance task. Metab Brain Dis 23:189–198
Acknowledgments
Our work is supported by the Swiss National Science Foundation, Grants 3100A0-116859 and 31003A-130278.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: T. Wallimann and R. Harris.
Rights and permissions
About this article
Cite this article
Hanna-El-Daher, L., Braissant, O. Creatine synthesis and exchanges between brain cells: What can be learned from human creatine deficiencies and various experimental models?. Amino Acids 48, 1877–1895 (2016). https://doi.org/10.1007/s00726-016-2189-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-016-2189-0