Abstract
Objective
Dynamic susceptibility contrast MRI (DSC-MRI) tends to return elevated estimates of cerebral blood flow (CBF) and cerebral blood volume (CBV). In this study, subject-specific calibration factors (CFs), based on steady-state CBV measurements, were applied to rescale the absolute level of DSC-MRI CBF.
Materials and methods
Twenty healthy volunteers were scanned in a test–retest approach. Independent CBV measurements for calibration were accomplished using a T1-based contrast agent steady-state method (referred to as Bookend), as well as a blood-nulling vascular space occupancy (VASO) approach. Calibrated DSC-MRI was compared with pseudo-continuous arterial spin labeling (pCASL).
Results
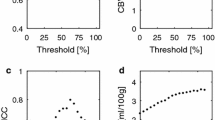
For segmented grey matter (GM) regions of interests (ROIs), pCASL-based CBF was 63 ± 11 ml/(min 100 g) (mean ± SD). Nominal CBF from non-calibrated DSC-MRI was 277 ± 61 ml/(min 100 g), while calibrations resulted in 56 ± 23 ml/(min 100 g) (Bookend) and 52 ± 16 ml/(min 100 g) (VASO). Calibration tended to eliminate the overestimation, although the repeatability was generally moderate and the correlation between calibrated DSC-MRI and pCASL was low (r < 0.25). However, using GM instead of WM ROIs for extraction of CFs resulted in improved repeatability.
Conclusion
Both calibration approaches provided reasonable absolute levels of GM CBF, although the calibration methods suffered from low signal-to-noise ratio, resulting in weak repeatability and difficulties in showing high degrees of correlation with pCASL measurements.







Similar content being viewed by others
References
Knutsson L, Ståhlberg F, Wirestam R (2010) Absolute quantification of perfusion using dynamic susceptibility contrast MRI: pitfalls and possibilities. Magn Reson Mater Phy 23:1–21
Baron JC (2001) Perfusion thresholds in human cerebral ischemia: historical perspective and therapeutic implications. Cerebrovasc Dis 11:2–8
Weber MA, Zoubaa S, Schlieter M, Jüttler E, Huttner HB, Geletneky K, Ittrich C, Lichy MP, Kroll A, Debus J, Giesel FL, Hartmann M, Essig M (2006) Diagnostic performance of spectroscopic and perfusion MRI for distinction of brain tumours. Neurology 66:1899–1906
Broich K, Hartmann A, Adam S, Biersack HJ (1989) Regional cerebral blood flow in dementia of Alzheimer’s type. J Neural Transm 1:41
Ishii K (2002) Clinical application of positron emission tomography for diagnosis of dementia. Ann Nucl Med 16:515–525
Ohgami H, Nagayama H, Akiyoshi J, Tsuchiyama K, Komaki S, Takaki H, Mori H (2005) Contributing factors to changes of cerebral blood flow in major depressive disorder. J Affect Disord 87:57–63
Shin W, Cashen TA, Horowitz SW, Sawlani R, Carroll TJ (2006) Quantitative CBV measurement from static T1 changes in tissue and correction for intravascular water exchange. Magn Reson Med 56:138–145
Sakaie KE, Shin W, Curtin KR, McCarthy RM, Cashen TA, Carroll TJ (2005) Method for improving the accuracy of quantitative cerebral perfusion imaging. J Magn Reson Imaging 21:512–519
Vakil P, Lee JJ, Mouannes-Srour JJ, Derdeyn CP, Carroll TJ (2013) Cerebrovascular occlusive disease: quantitative cerebral blood flow using dynamic susceptibility contrast MR imaging correlates with quantitative H2[15O] PET. Radiology 266:879–886
Lu H, Law M, Johnson G, Ge Y, van Zijl PC, Helpern JA (2005) Novel approach to the measurement of absolute cerebral blood volume using vascular-space-occupancy magnetic resonance imaging. Magn Reson Med 54:1403–1411
Xu G, Rowley HA, Wu G, Alsop DC, Shankaranarayanan A, Dowling M, Christian BT, Oakes TR, Johnson SC (2010) Reliability and precision of pseudo-continuous arterial spin labeling perfusion MRI on 3.0 T and comparison with 15O-water PET in elderly subjects at risk for Alzheimer’s disease. NMR Biomed 23:286–293
Ye FQ, Berman KF, Ellmore T, Eposito G, van Horn JD, Yang Y, Duyn J, Smith AM, Frank JA, Weinberger DR, McLaughlin AC (2000) H 152 O PET validation of steady-state arterial spin tagging cerebral blood flow measurements in humans. Magn Reson Med 44:450–456
Kuppusamy K, Lin W, Cizek GR, Haacke EM (1996) In vivo regional cerebral blood volume: quantitative assessment with 3D T1-weighted pre- and postcontrast MR imaging. Radiology 201:106–112
Hazlewood CF, Chang DC, Nichols BL, Woessner DE (1974) Nuclear magnetic resonance transverse relaxation times of water protons in skeletal muscle. Biophys J 14:583–606
Donahue KM, Weisskoff RM, Chesler DA, Kwong KK, Bogdanov AA Jr, Mandeville JB, Rosen BR (1996) Improving MR quantification of regional blood volume with intravascular T1 contrast agents: accuracy, precision, and water exchange. Magn Reson Med 36:858–867
Srour JM, Shin W, Shah S, Sen A, Carroll TJ (2011) SCALE-PWI: a pulse sequence for absolute quantitative cerebral perfusion imaging. J Cereb Blood Flow Metab 31:1272–1282
Alsop DC, Detre JA (1996) Reduced transit-time sensitivity in noninvasive magnetic resonance imaging of human cerebral blood flow. J Cereb Blood Flow Metab 16:1236–1249
Wang J, Alsop DC, Li L, Listerud J, Gonzalez-At JB, Schnall MD, Detre JA (2002) Comparison of quantitative perfusion imaging using arterial spin labeling at 1.5 and 4.0 Tesla. Magn Reson Med 48:242–254
Young S, Bystrov D, Netsch T, Bergmans R, van Muiswinkel A, Visser F, Springorum R, Gieseke J (2006) Automated planning of MRI neuro scans. In: Proceedings of the SPIE 6144 on medical imaging (image processing). San Diego, 61441M
Look DC, Locker DR (1970) Time saving in measurement of NMR and EPR relaxation times. Rev Sci Instrum 41:250–251
Rempp KA, Brix G, Wenz F, Becker CR, Gückel F, Lorenz WJ (1994) Quantification of regional cerebral blood flow and volume with dynamic susceptibility contrast-enhanced MR imaging. Radiology 193:637–641
Wu O, Østergaard L, Weisskoff RM, Benner T, Rosen BR, Sorensen AG (2003) Tracer arrival timing-insensitive technique for estimating flow in MR perfusion-weighted imaging using singular value decomposition with a block-circulant deconvolution matrix. Magn Reson Med 50:164–174
Messroghli DR, Rudolph A, Abdel-Aty H, Wassmuth R, Kühne T, Dietz R, Schulz-Menger J (2010) An open-source software tool for the generation of relaxation time maps in magnetic resonance imaging. BMC Med Imaging 10:16
Lu H, Clingman C, Golay X, van Zijl PC (2004) Determining the longitudinal relaxation time (T1) of blood at 3.0 Tesla. Magn Reson Med 52:679–682
Uh J, Lewis-Amezcua K, Varghese R, Lu H (2009) On the measurement of absolute cerebral blood volume (CBV) using vascular-space-occupancy (VASO) MRI. Magn Reson Med 61:659–667
van Osch MJ, Teeuwisse WM, van Walderveen MA, Hendrikse J, Kies DA, van Buchem MA (2009) Can arterial spin labeling detect white matter perfusion signal? Magn Reson Med 62:165–173
Donahue MJ, Lu H, Jones CK, Edden RA, Pekar JJ, van Zijl PC (2006) Theoretical and experimental investigation of the VASO contrast mechanism. Magn Reson Med 56:1261–1273
Cavuşoğlu M, Pfeuffer J, Uğurbil K, Uludağ K (2009) Comparison of pulsed arterial spin labeling encoding schemes and absolute perfusion quantification. Magn Reson Imaging 27:1039–1045
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 327:307–310
Petersen ET, Mouridsen K, Golay X, all named co-authors of the Quasar test-retest study (2010) The QUASAR reproducibility study, Part II: results from a multi-center Arterial Spin Labeling test-retest study. Neuroimage 49:104–113
Leenders KL, Perani D, Lammertsma AA, Heather JD, Buckingham P, Healy MJR, Gibbs JM, Wise RJS, Hatazawa J, Herold S, Beaney RP, Brooks DJ, Spinks T, Rhodes C, Frackowiak RSJ, Jones T (1990) Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain 113:27–47
Meltzer CC, Cantwell MN, Greer PJ, Ben-Eliezer D, Smith G, Frank G, Kaye WH, Houck PR, Price JC (2000) Does cerebral blood flow decline in healthy aging? A PET study with partial-volume correction. J Nucl Med 41:1842–1848
Ito H, Kanno I, Ibaraki M, Hatazawa J, Miura S (2003) Changes in human cerebral blood flow and cerebral blood volume during hypercapnia and hypocapnia measured by positron emission tomography. J Cereb Blood Flow Metab 23:665–670
Yamaguchi T, Kanno I, Uemura K, Shishido F, Inugami A, Ogawa T, Murakami M, Suzuki K (1986) Reduction in regional cerebral metabolic rate of oxygen during human aging. Stroke 17:1220–1228
Larsson HBW, Fritz-Hansen T, Rostrup E, Søndergaard L, Ring P, Henriksen O (1996) Myocardial perfusion modeling using MRI. Magn Reson Med 35:716–726
Shin W, Horowitz S, Ragin A, Chen Y, Walker M, Carroll TJ (2007) Quantitative cerebral perfusion using dynamic susceptibility contrast MRI: evaluation of reproducibility and age- and gender-dependence with fully automatic image postprocessing algorithm. Magn Reson Med 58:1232–1241
Crane DE, Donahue MJ, Chappell MA, Sideso E, Handa A, Kennedy J, Jezzard P, MacIntosh BJ (2013) Evaluating quantitative approaches to dynamic susceptibility contrast MRI among carotid endarterectomy patients. J Magn Reson Imaging 37:936–943
Acknowledgments
Thanks to Dr. Christian Stehning at Philips Research in Hamburg for providing the sequence for T1 measurements. This study was supported by the Swedish Research Council (Grant Nos. 13514 and 2010-4454).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lindgren, E., Wirestam, R., Markenroth Bloch, K. et al. Absolute quantification of perfusion by dynamic susceptibility contrast MRI using Bookend and VASO steady-state CBV calibration: a comparison with pseudo-continuous ASL. Magn Reson Mater Phy 27, 487–499 (2014). https://doi.org/10.1007/s10334-014-0431-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-014-0431-x