Abstract
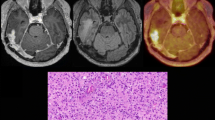
Objective To explore prospectively the positive predictive value of O-(2-[18F]fluoroethyl)-l-tyrosine (FET)–PET in selected patients with a magnetic resonance imaging (MRI)-based suspicion of a glioma recurrence or progression. Methods Patients with a supratentorial glioma (initial World Health Organization (WHO) grade II, III or IV) were considered eligible if they had both an MRI-(new/progressive contrast-enhancing lesion) and FET–PET-based diagnosis of a recurrence/progression after various forms and combinations of irradiation and chemotherapy. Criterion for tumour recurrence/progression in FET–PET was a standardized uptake value (SUVmax)/Background (BG) ratio of >2.0 in the late uptake phase. All patients underwent multimodal (MRI, FET–PET) imaging-guided stereotactic biopsy. The positive predictive value was defined as the proportion of MRI and FET–PET findings indicating glioma recurrence/progression that also tested positive for tumour recurrence/progression after stereotactic biopsy. Results Thirty-one patients with initially WHO grade II (17), WHO grade III (6), and grade IV glioma (8) were included. In 26 patients FET–PET results indicating tumour recurrence/progression were concordant with the biopsy results. In five patients histopathologic evaluation failed to reveal a “vital” tumour. FET–PET findings were also discordant with the radiographic and clinical follow-up in these five patients. The positive predictive value of FET–PET was 84%. Conclusion The positive predictive value of FET–PET using the standard ratio method is high, but not high enough to replace stereotactic biopsy in this highly selected study cohort. Whether the calculation of FET uptake in the early phase and/or the evaluation of uptake kinetics will improve the positive predictive value of FET–PET deserves prospective evaluation.

Similar content being viewed by others
References
Dooms GC, Hecht S, Brant-Zawadzki M et al (1986) Brain radiation lesions: MR imaging. Radiology 158:149–155
Floeth FW, Wittsack HJ, Engelbrecht V et al (2002) Comparative follow-up of enhancement phenomena with MRI and Proton MR Spectroscopic Imaging after intralesional immunotherapy in glioblastoma—report of two exceptional cases. Zentralbl Neurochir 63:23–28
Kumar AJ, Leeds NE, Fuller GN et al (2000) Malignant gliomas: MR Imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology 217:377–384
Ross DA, Sandler HM, Balter JM et al (2002) Imaging changes after stereotactic radiosurgery of primary and secondary malignant brain tumours. J Neurooncol 56:175–181
Chang EL, Loeffler JS, Riese NE et al (1998) Survival results from a phase I study of etonidazole and radiotherapy in patients with malignant glioma. Int J Radiat Oncol Biol Phys 40:65–70
Floeth FW, Shand N, Bojar H et al (2002) Local inflammation and devascularization—in vivo mechanisms of the “bystander effect” in VPC-mediated HSV-Tk/GCV gene therapy for human malignant glioma. Cancer Gene Ther 8:843–851
Goetz C, Riva P, Poepperl G et al (2003) Locoregional radioimmunotherapy in selected patients with malignant glioma: experiences, side effects and survival times. J Neurooncol 62:321–328
Kreth FW, Faist M, Warnke PC et al (1995) Interstitial radiosurgery of low-grade gliomas. J Neurosurg 82:418–429
Kreth FW, Warnke PC, Scheremet R et al (1993) Surgical resection and radiation therapy versus biopsy and radiation therapy in the treatment of glioblastoma multiforme. J Neurosurg 78:762–766
Mardor Y, Roth Y, Lidar Z et al (2001) Monitoring response to convection-enhanced Taxol delivery in brain tumour patients using diffusion-weighted magnetic resonance imaging. Cancer Res 61:4971–4973
Westphal M, Hilt DC, Bortey E et al (2003) A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol 5:79–88
Westphal M, Tonn JC, Ram Z (eds) (2003) Local therapies for glioma: present status and future developments. Acta Neurochir Suppl, Springer, Vienna
Stenberg L, Englund E, Wirestam R et al (2006) Dynamic susceptibility contrast-enhanced perfusion magnetic resonance (MR) imaging combined with contrast-enhanced MR imaging in the follow-up of immunogene-treated glioblastoma multiforme. Acta Radiol 47:852–861
Zeng QS, Li CF, Liu H et al (2007) Distinction between recurrent glioma and radiation injury using magnetic resonance spectroscopy in combination with diffusion-weighted imaging. Int J Radiat Oncol Biol Phys 68:151–158. Epub 2007 Feb 7
Poepperl G, Götz C, Rachinger W et al (2004) Value of O-(2-[18F]fluoroethyl)-l-tyrosine PET for the diagnosis of recurrent glioma. Eur J Nucl Med Mol Imaging 31:1464–1470
Rachinger W, Goetz C, Poepperl G et al (2005) Positron emission tomography with O-(2-[18F]fluoroethyl)-l-tyrosine versus magnetic resonance imaging in the diagnosis of recurrent gliomas. Neurosurgery 57:505–511
Langen KJ, Hamacher K, Weckesser M et al (2006) O-(2-[18F]fluoroethyl)-l-tyrosine: uptake mechanisms and clinical applications. Nucl Med Biol 33:287–294
Olivero WC, Dulebohn SC, Lister JR (1995) The use of PET in evaluating patients with primary brain tumours: is it useful? J Neurol Neurosurg Psychiatry 58:250–252
Ricci PE, Karis JP, Heiserman JE, Fram EK et al (1998) Differentiating recurrent tumor from radiation necrosis: time for re-evaluation of positron emission tomography? AJNR Am J Neuroradiol 19:407–413
Langleben DD, Segall GM (2000) PET in differentiation of recurrent brain tumor from radiation injury. J Nucl Med 41:1861–1867
Weber WA, Wester HJ, Grosu A et al (2000) O-(2-[18F]Fluoroethyl)-l-tyrosine and l-[methyl-11C]methionine uptake in brain tumours: initial results of a comparative study. Eur J Nucl Med 27:542–549
Wester HJ, Herz M, Weber W et al (1999) Synthesis and radiopharmacology of O-(2-[18F]fluorethyl)-l-tyrosine for tumor imaging. J Nucl Med 40:205–212
Poepperl G, Goldbrunner R, Gildehaus FJ et al (2005) O-(2-[18F]fluoroethyl)-l-tyrosine PET for monitoring the effects of convection-enhanced delivery of paclitaxel in patients with recurrent glioblastoma. Eur J Nucl Med Mol Imaging 32:1018–1025
Poepperl G, Kreth FW, Herms J et al (2006) Analysis of 18F-FET–PET for grading of recurrent gliomas: is evaluation of uptake kinetics superior to standard methods? J Nucl Med 47:393–403
Floeth FW, Pauleit D, Wittsack HJ et al (2005) Multimodal metabolic imaging of cerebral gliomas: positron emission tomography with [18F]fluoroethyl-l-tyrosine and magnetic resonance spectroscopy. J Neurosurg 102:318–327
Pauleit D, Floeth F, Hamacher K et al (2005) O-(2-[18F]fluoroethyl)-l-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain 128:678–687
Kreth FW, Muacevic A, Medele R et al (2001) The risk of hemorrhage after image guided stereotactic biopsy of intra-axial brain tumours—a retrospective study. Acta Neurochir 143:539–546
Kleihues P, Cavenee WK (2000) Tumours of the nervous system. Pathology and Genetics. IARC Press, Lyon, France
Poepperl G, Goetz C, Gildehaus FJ et al (2002) Initial experiences with adjuvant locoregional radioimmunotherapy using 131I-labeled monoclonal antibodies against tenascin (BC-4) for treatment of glioma (WHO III and IV). Nuklearmedizin 41:120–128
Beck TJ, Kreth FW, Beyer W et al (2007) Interstitial photodynamic therapy of nonresectable malignant glioma recurrences using 5-aminolevulinic acid induced protoporphyrin IX. Lasers Surg Med 39:386–393
Forsyth PA, Kelly PJ, Cascino TL et al (1995) Radiation necrosis or glioma recurrence: is computer-assisted stereotactic biopsy useful? J Neurosurg 82:436–444
Kreth FW, Muacevic A (1999) Stereotactic biopsy and hemorrhage. J Neurosurg 90:181–182
Thompson TP, Lunsford LD, Kondziolka D (1999) Distinguishing recurrent tumour and radiation necrosis with positron emission tomography versus stereotactic biopsy. Stereotact Funct Neurosurg 73:9–14
Kracht LW, Friese M, Herholz K et al (2003) Methyl-[(11)C]-l-methionine uptake as measured by positron emission tomography correlates to microvessel density in patients with glioma. Eur J Nucl Med Mol Imaging 30:868–873
Fortin D, Cairncross GJ, Hammond RR (1999) Oligodendroglioma: an appraisal of recent data pertaining to diagnosis and treatment. Neurosurgery 45:1279–1291; discussion 1191
Floeth FW, Pauleit D, Sabel M et al (2006) 18F-FET–PET differentiation of ring-enhancing brain lesions. J Nucl Med 47:776–782
Ostertag CB, Weigel K, Warnke P et al (1983) Sequential morphological changes in the dog brain after interstitial iodine-125 irradiation. Neurosurgery 13:523–528
Ceyssens S, Van Laere K, de Groot T et al (2006) [11C]methionine PET, histopathology, and survival in primary brain tumors and recurrence. AJNR Am J Neuroradiol 27:1432–1437
Weckesser M, Langen KJ, Rickert CH et al (2005) O-(2-[(18)F]fluorethyl)-l-tyrosine PET in the clinical evaluation of primary brain tumours. Eur J Nucl Med Mol Imaging 32:422–429
Pirotte B, Goldman S, Massager N et al (2004) Comparison of 18F-FDG and 11C-methionine for PET-guided stereotactic brain biopsy of gliomas. J Nucl Med 45:1293–1298
Acknowledgements
Part of this work was supported by grant 10-3163-Wi3 from Deutsche Krebshilfe.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jan-Hinnerk Mehrkens and Gabriele Pöpperl have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Mehrkens, J.H., Pöpperl, G., Rachinger, W. et al. The positive predictive value of O-(2-[18F]fluoroethyl)-l-tyrosine (FET) PET in the diagnosis of a glioma recurrence after multimodal treatment. J Neurooncol 88, 27–35 (2008). https://doi.org/10.1007/s11060-008-9526-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-008-9526-4