Abstract
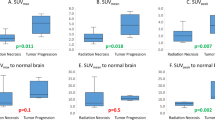
To study the ability of dual phase FDG-PET/CT imaging to accurately distinguish tumor versus necrosis in patients treated for brain metastases. 32 (22 female, 10 male) consecutive patients with treated brain metastases, lesion size greater than 0.5 cm3 and suspected recurrence on MRI underwent dual-phase FDG-PET/CT. Clinical outcome was assessed by biopsy or by MRI. SUVmax and SUVmean values of the lesion (L) and gray matter (GM) at the level of the thalamus were measured on early (1) and delayed (2) imaging. L1/GM1 and L2/GM2 and the change of L/GM ratios as a function of time were calculated [(L2/GM2 − L1/GM1)/(L1/GM1)]. Cut-off values were obtained by ROC analysis. P < 0.05 defined statistical significance. Seven patients were excluded due to indeterminate outcomes. 25 patients (16 female, 9 male; 27 lesions; 28 scan sessions) had clear outcomes, proven by either biopsy (n = 16 patients) or serial follow-up MRI (n = 9 patients). Primary subtypes included breast (n = 9), lung (n = 7), melanoma (n = 3), squamous cell cancer of the head and neck (n = 2) and other (n = 4). Twenty-two patients underwent prior radiation (2–113 months) and three received only prior chemotherapy (5 months to 3 years). A change >0.19 of L/GM ratios as a function of time was 95% sensitive, 100% specific, and 96.4% accurate (P = 0.0001; AUC = 0.97) for distinguishing tumor versus radiation necrosis. The ratio of the change of the lesion to WM ratios over time was the second best indicator of outcome when compared to all indices used (ROC cut-off = 0.25, sensitivity 89.5% and specificity 90.9%, and accuracy 89.2%; P = 0.0001; AUC = 0.95), Early or late SUVs of the lesion alone did not differentiate between tumor and necrosis. Regardless of histological type, differentiation of necrosis from metastatic brain lesions was improved by using the change of lesion to gray matter SUVmax ratios as a function of time.






Similar content being viewed by others
References
Zhuang H et al (2001) Dual time point 18F-FDG PET imaging for differentiating malignant from inflammatory processes. J Nucl Med 42(9):1412–1417
Waki A, Fujibayashi Y, Yokoyama A (1998) Recent advances in the analyses of the characteristics of tumors on FDG uptake. Nucl Med Biol 25(7):589–592
Kubota K et al (2001) Advantage of delayed whole-body FDG-PET imaging for tumour detection. Eur J Nucl Med 28(6):696–703
Hustinx R et al (1999) Dual time point fluorine-18 fluorodeoxyglucose positron emission tomography: a potential method to differentiate malignancy from inflammation and normal tissue in the head and neck. Eur J Nucl Med 26(10):1345–1348
Gerbaudo VH et al (2003) Metabolic significance of the pattern, intensity and kinetics of 18F-FDG uptake in malignant pleural mesothelioma. Thorax 58(12):1077–1082
Spence AM et al (2004) 18F-FDG PET of gliomas at delayed intervals: improved distinction between tumor and normal gray matter. J Nucl Med 45(10):1653–1659
Ishizu K et al (1994) Effects of hyperglycemia on FDG uptake in human brain and glioma. J Nucl Med 35(7):1104–1109
Delbeke D et al (1995) Optimal cutoff levels of F-18 fluorodeoxyglucose uptake in the differentiation of low-grade from high-grade brain tumors with PET. Radiology 195(1):47–52
Britz-Cunningham SH, Millstine JW, Gerbaudo VH (2008) Improved discrimination of benign and malignant lesions on FDG PET/CT, using comparative activity ratios to brain, basal ganglia, or cerebellum. Clin Nucl Med 33(10):681–687
Chen W et al (2006) 18F-FDOPA PET imaging of brain tumors: comparison study with 18F-FDG PET and evaluation of diagnostic accuracy. J Nucl Med 47(6):904–911
Gonzalez J et al (2007) Effect of bevacizumab on radiation necrosis of the brain. Int J Radiat Oncol Biol Phys 67(2):323–326
Arnold SM, Patchell RA (2001) Diagnosis and management of brain metastases. Hematol Oncol Clin North Am 15(6):1085–1107
Hustinx R et al (2005) PET imaging for differentiating recurrent brain tumor from radiation necrosis. Radiol Clin North Am 43(1):35–47
Langleben DD, Segall GM (2000) PET in differentiation of recurrent brain tumor from radiation injury. J Nucl Med 41(11):1861–1867
Patronas NJ et al (1982) Work in progress: [18F] fluorodeoxyglucose and positron emission tomography in the evaluation of radiation necrosis of the brain. Radiology 144(4):885–889
Gambhir SS et al (2001) A tabulated summary of the FDG PET literature. J Nucl Med 42(5 Suppl):1S–93S
Mogard J et al (1994) Recurrent tumor vs radiation effects after gamma knife radiosurgery of intracerebral metastases: diagnosis with PET-FDG. J Comput Assist Tomogr 18(2):177–181
Ricci PE et al (1998) Differentiating recurrent tumor from radiation necrosis: time for re-evaluation of positron emission tomography? AJNR Am J Neuroradiol 19(3):407–413
Chen W et al (2005) Imaging proliferation in brain tumors with 18F-FLT PET: comparison with 18F-FDG. J Nucl Med 46(6):945–952
Kang TW et al (2009) Morphological and functional MRI, MRS, perfusion and diffusion changes after radiosurgery of brain metastasis. Eur J Radiol 72(3):370–380
Sokoloff L et al (1977) The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem 28(5):897–916
Spence AM et al (2004) 18F-FDG PET of gliomas at delayed intervals: improved distinction between tumor and normal gray matter. J Nucl Med 45(10):1653–1659
Bissonnette P et al (1996) 2-Deoxyglucose transport and metabolism in Caco-2 cells. Am J Physiol 270(1 Pt 1):G153–G162
Higashi T et al (2002) Relationship between retention index in dual-phase (18)F-FDG PET, and hexokinase-II and glucose transporter-1 expression in pancreatic cancer. J Nucl Med 43(2):173–180
Lodge MA et al (1999) A PET study of 18FDG uptake in soft tissue masses. Eur J Nucl Med 26(1):22–30
Yamada S et al (1995) High accumulation of fluorine-18-fluorodeoxyglucose in turpentine-induced inflammatory tissue. J Nucl Med 36(7):1301–1306
Hamberg LM et al (1994) The dose uptake ratio as an index of glucose metabolism: useful parameter or oversimplification? J Nucl Med 35(8):1308–1312
Zhao (2003) Delayed 18F-FDG PET brain imaging improves detection rate of brain metastases. In: Proceedings of the SNM 50th annual meeting, vol 44(5), p 243P
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Horky, L.L., Hsiao, E.M., Weiss, S.E. et al. Dual phase FDG-PET imaging of brain metastases provides superior assessment of recurrence versus post-treatment necrosis. J Neurooncol 103, 137–146 (2011). https://doi.org/10.1007/s11060-010-0365-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-010-0365-8