Abstract
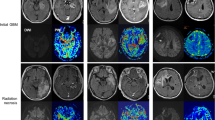
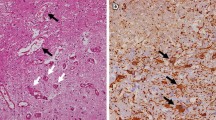
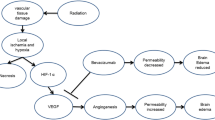
The cell type and localization of vascular endothelial growth factor (VEGF)-producing cells in human radiation necrosis (RN) are investigated from a histopathological and immunohistochemical standpoint using clinical specimens. Eighteen surgical specimens of symptomatic RN in the brain were retrospectively reviewed. These cases included different original histological tumor types and were treated with different radiation modalities. Histological analyses were performed using hematoxylin and eosin (H&E) staining, and anti-VEGF and anti-hypoxia-inducible factor (HIF)-1α immunohistochemistry. H&E staining showed marked angiogenesis and reactive astrocytosis at the perinecrotic area. The most prominent vasculature in this area was identified as telangiectasis. Immunohistochemistry indicated that HIF-1α was expressed predominantly in the perinecrotic area and that a large majority of VEGF-expressing cells were reactive astrocytes intensively distributed in this area. VEGF produced by the reactive astrocytes localized mainly in the perinecrotic area might be a major cause of both angiogenesis and the subsequent perilesional edema typically found in RN of the brain. The benefits of anti-VEGF antibody (bevacizumab) treatment in RN may be that VEGF secretion from the perinecrotic tissue is inhibited and that surgery would remove this tissue; both of these benefits result in effective reduction of edema associated with RN.




Similar content being viewed by others
References
Hopewell JW (1998) Radiation injury to the central nervous system. Med Pediatr Oncol Suppl 1:1–9
Fitzek MM, Thornton AF, Rabinov JD, Lev MH, Pardo FS, Munzenrider JE, Okunieff P, Bussiere M, Braun I, Hochberg FH, Hedley-Whyte ET, Liebsch NJ, Harsh GRt (1999) Accelerated fractionated proton/photon irradiation to 90 cobalt gray equivalent for glioblastoma multiforme: results of a phase II prospective trial. J Neurosurg 91:251–260
Iuchi T, Hatano K, Narita Y, Kodama T, Yamaki T, Osato K (2006) Hypofractionated high-dose irradiation for the treatment of malignant astrocytomas using simultaneous integrated boost technique by IMRT. Int J Radiat Oncol Biol Phys 64:1317–1324
Kawabata S, Miyatake S, Kuroiwa T, Yokoyama K, Doi A, Iida K, Miyata S, Nonoguchi N, Michiue H, Takahashi M, Inomata T, Imahori Y, Kirihata M, Sakurai Y, Maruhashi A, Kumada H, Ono K (2009) Boron neutron capture therapy for newly diagnosed glioblastoma. J Radiat Res 50:51–60
Miyatake S, Kawabata S, Kajimoto Y, Aoki A, Yokoyama K, Yamada M, Kuroiwa T, Tsuji M, Imahori Y, Kirihata M, Sakurai Y, Masunaga S, Nagata K, Maruhashi A, Ono K (2005) Modified boron neutron capture therapy for malignant gliomas performed using epithermal neutron and two boron compounds with different accumulation mechanisms: an efficacy study based on findings on neuroimages. J Neurosurg 103:1000–1009
Tanaka M, Ino Y, Nakagawa K, Tago M, Todo T (2005) High-dose conformal radiotherapy for supratentorial malignant glioma: a historical comparison. Lancet Oncol 6:953–960
Ohguri T, Imada H, Kohshi K, Kakeda S, Ohnari N, Morioka T, Nakano K, Konda N, Korogi Y (2007) Effect of prophylactic hyperbaric oxygen treatment for radiation-induced brain injury after stereotactic radiosurgery of brain metastases. Int J Radiat Oncol Biol Phys 67:248–255
Guttin PH (1991) Treatment of radiation necrosis of the brain. Raven, New York
Leibel SA, Gutin PH, Wara WM, Silver PS, Larson DA, Edwards MS, Lamb SA, Ham B, Weaver KA, Barnett C et al (1989) Survival and quality of life after interstitial implantation of removable high-activity iodine-125 sources for the treatment of patients with recurrent malignant gliomas. Int J Radiat Oncol Biol Phys 17:1129–1139
Levin VA, Bidaut L, Hou P, Kumar AJ, Wefel JS, Bekele BN, Prabhu S, Loghin M, Gilbert MR, Jackson EF (2011) Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys 79(5):1487–1495
Gonzalez J, Kumar AJ, Conrad CA, Levin VA (2007) Effect of bevacizumab on radiation necrosis of the brain. Int J Radiat Oncol Biol Phys 67:323–326
Torcuator R, Zuniga R, Mohan YS, Rock J, Doyle T, Anderson J, Gutierrez J, Ryu S, Jain R, Rosenblum M, Mikkelsen T (2009) Initial experience with bevacizumab treatment for biopsy confirmed cerebral radiation necrosis. J Neurooncol 94:63–68
Furuse M, Kawabata S, Kuroiwa T, Miyatake SI (2010) Repeated treatments with bevacizumab for recurrent radiation necrosis in patients with malignant brain tumors: a report of 2 cases. J Neurooncol 102(3):471–475
Miyatake S, Kawabata S, Nonoguchi N, Yokoyama K, Kuroiwa T, Matsui H, Ono K (2009) Pseudoprogression in boron neutron capture therapy for malignant gliomas and meningiomas. Neuro Oncol 11:430–436
Miyashita M, Miyatake S, Imahori Y, Yokoyama K, Kawabata S, Kajimoto Y, Shibata MA, Otsuki Y, Kirihata M, Ono K, Kuroiwa T (2008) Evaluation of fluoride-labeled boronophenylalanine-PET imaging for the study of radiation effects in patients with glioblastomas. J Neurooncol 89:239–246
Stummer W, Novotny A, Stepp H, Goetz C, Bise K, Reulen HJ (2000) Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: a prospective study in 52 consecutive patients. J Neurosurg 93:1003–1013
Stummer W, Stocker S, Wagner S, Stepp H, Fritsch C, Goetz C, Goetz AE, Kiefmann R, Reulen HJ (1998) Intraoperative detection of malignant gliomas by 5-aminolevulinic acid-induced porphyrin fluorescence. Neurosurgery 42:518–525 discussion 525–516
Keck PJ, Hauser SD, Krivi G, Sanzo K, Warren T, Feder J, Connolly DT (1989) Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science 246:1309–1312
Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N (1989) Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246:1306–1309
Connolly DT, Heuvelman DM, Nelson R, Olander JV, Eppley BL, Delfino JJ, Siegel NR, Leimgruber RM, Feder J (1989) Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Invest 84:1470–1478
Connolly DT, Olander JV, Heuvelman D, Nelson R, Monsell R, Siegel N, Haymore BL, Leimgruber R, Feder J (1989) Human vascular permeability factor. Isolation from U937 cells. J Biol Chem 264:20017–20024
Zacchigna S, Tasciotti E, Kusmic C, Arsic N, Sorace O, Marini C, Marzullo P, Pardini S, Petroni D, Pattarini L, Moimas S, Giacca M, Sambuceti G (2007) In vivo imaging shows abnormal function of vascular endothelial growth factor-induced vasculature. Hum Gene Ther 18:515–524
Kimura R, Nakase H, Tamaki R, Sakaki T (2005) Vascular endothelial growth factor antagonist reduces brain edema formation and venous infarction. Stroke 36:1259–1263
van Bruggen N, Thibodeaux H, Palmer JT, Lee WP, Fu L, Cairns B, Tumas D, Gerlai R, Williams SP, van Lookeren Campagne M, Ferrara N (1999) VEGF antagonism reduces edema formation and tissue damage after ischemia/reperfusion injury in the mouse brain. J Clin Invest 104:1613–1620
Li YQ, Ballinger JR, Nordal RA, Su ZF, Wong CS (2001) Hypoxia in radiation-induced blood-spinal cord barrier breakdown. Cancer Res 61:3348–3354
Nordal RA, Nagy A, Pintilie M, Wong CS (2004) Hypoxia and hypoxia-inducible factor-1α target genes in central nervous system radiation injury: a role for vascular endothelial growth factor. Clin Cancer Res 10:3342–3353
Minchenko A, Bauer T, Salceda S, Caro J (1994) Hypoxic stimulation of vascular endothelial growth factor expression in vitro and in vivo. Lab Invest 71:374–379
Fang J, Yan L, Shing Y, Moses MA (2001) HIF-1alpha-mediated up-regulation of vascular endothelial growth factor, independent of basic fibroblast growth factor, is important in the switch to the angiogenic phenotype during early tumorigenesis. Cancer Res 61:5731–5735
Ikeda E, Achen MG, Breier G, Risau W (1995) Hypoxia-induced transcriptional activation and increased mRNA stability of vascular endothelial growth factor in C6 glioma cells. J Biol Chem 270:19761–19766
Shweiki D, Itin A, Soffer D, Keshet E (1992) Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 359:843–845
Kalaria RN, Cohen DL, Premkumar DR, Nag S, LaManna JC, Lust WD (1998) Vascular endothelial growth factor in Alzheimer’s disease and experimental cerebral ischemia. Brain Res Mol Brain Res 62:101–105
Miyatake S, Kuroiwa T, Kajimoto Y, Miyashita M, Tanaka H, Tsuji M (2007) Fluorescence of non-neoplastic, magnetic resonance imaging-enhancing tissue by 5-aminolevulinic acid: case report. Neurosurgery 61:E1101–E1103 discussion E1103–1104
Calvo W, Hopewell JW, Reinhold HS, Yeung TK (1988) Time- and dose-related changes in the white matter of the rat brain after single doses of X rays. Br J Radiol 61:1043–1052
Burger PC, Mahley MS Jr, Dudka L, Vogel FS (1979) The morphologic effects of radiation administered therapeutically for intracranial gliomas: a postmortem study of 25 cases. Cancer 44:1256–1272
Gaensler EH, Dillon WP, Edwards MS, Larson DA, Rosenau W, Wilson CB (1994) Radiation-induced telangiectasia in the brain simulates cryptic vascular malformations at MR imaging. Radiology 193:629–636
Poussaint TY, Siffert J, Barnes PD, Pomeroy SL, Goumnerova LC, Anthony DC, Sallan SE, Tarbell NJ (1995) Hemorrhagic vasculopathy after treatment of central nervous system neoplasia in childhood: diagnosis and follow-up. AJNR Am J Neuroradiol 16:693–699
Rabin BM, Meyer JR, Berlin JW, Marymount MH, Palka PS, Russell EJ (1996) Radiation-induced changes in the central nervous system and head and neck. Radiographics 16:1055–1072
Burger P, Boyko O (1991) The pathology of central nervous system radiation injury. In: Gutin PH, Leibel SA, Sheline GE (eds) Radiation injury to the nervous system. Raven Press, New York, pp 191–208
Acknowledgments
The first two authors contributed equally to this work. This work was partly supported by Grants-in-Aid for Scientific Research (B) (16390422 and 19390385) from the Japanese Ministry of Education, Culture, Sports, Science, and Technology to S.-I.M. This work was also supported in part by the Takeda Science Foundation for Osaka Medical College and in part by a grant from the OMC Science Frontier Program for the Promotion of Research in Osaka Medical College to S.-I.M. We appreciate the help of Dr. Shingo Takano, Department of Neurosurgery, Tsukuba University, for providing information on immunohistochemistry of hypoxia-inducible factor-1α; and the help of Hiroko Kuwabara, Department of Pathology, Osaka Medical College, for fruitful discussion on the histological findings of the pathological specimens.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nonoguchi, N., Miyatake, SI., Fukumoto, M. et al. The distribution of vascular endothelial growth factor-producing cells in clinical radiation necrosis of the brain: pathological consideration of their potential roles. J Neurooncol 105, 423–431 (2011). https://doi.org/10.1007/s11060-011-0610-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-011-0610-9