Abstract
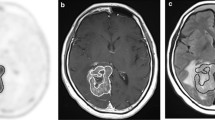
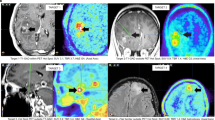
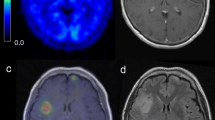
Pretreatment delineation of infiltrating glioma volume remains suboptimal with current neuroimaging techniques. Gadolinium-enhanced T1-weighted (T1-Gad) MR images often underestimate the true extent of the tumor, while T2-weighted images preferentially highlight peritumoral edema. Accumulation of α-[11C]methyl-l-tryptophan (AMT) on positron emission tomography (PET) has been shown in gliomas. To determine whether increased uptake on AMT–PET would detect tumor-infiltrated brain tissue outside the contrast-enhancing region and differentiate it from peritumoral vasogenic edema, volumes and spatial concordance of T1-Gad and T2 MRI abnormalities as well as AMT–PET abnormalities were analyzed in 28 patients with newly-diagnosed WHO grade II–IV gliomas. AMT-accumulating grade I meningiomas were used to define an AMT uptake cutoff threshold that detects the tumor but excludes peri-meningioma vasogenic edema. Tumor infiltration in AMT-accumulating areas was studied in stereotactically-resected specimens from patients with glioblastoma. In the 28 gliomas, mean AMT–PET-defined tumor volumes were greater than the contrast-enhancing volume, but smaller than T2 abnormalities. Volume of AMT-accumulating tissue outside MRI abnormalities increased with higher tumor proliferative index and was the largest in glioblastomas. Tumor infiltration was confirmed by histopathology from AMT-positive regions outside contrast-enhancing glioblastoma mass, while no or minimal tumor cells were found in AMT-negative specimens. These results demonstrate that increased AMT accumulation on PET detects glioma-infiltrated brain tissue extending beyond the contrast-enhanced tumor mass. While tryptophan uptake is low in peritumoral vasogenic edema, AMT–PET can detect tumor-infiltrated brain outside T2-lesions. Thus, AMT–PET may assist pretreatment delineation of tumor infiltration, particularly in high-grade gliomas.




Similar content being viewed by others
References
Pouratian N, Schiff D (2010) Management of low-grade glioma. Curr Neurol Neurosci Rep 10:224–231. doi:10.1007/s11910-010-0105-7
Mittal S, Szlaczky MC, Barger GR (2008) Low-grade gliomas in adults. Curr Treat Options Neurol 10:271–284
Swanson KR, Alvord EC Jr, Murray JD (2002) Virtual brain tumours (gliomas) enhance the reality of medical imaging and highlight inadequacies of current therapy. Br J Cancer 86:14–18. doi:10.1038/sj.bjc.6600021
Wallner KE, Galicich JH, Krol G, Arbit E, Malkin MG (1989) Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int J Radiat Oncol Biol Phys 16:1405–1409
Liang BC, Thornton AF Jr, Sandler HM, Greenberg HS (1991) Malignant astrocytomas: focal tumor recurrence after focal external beam radiation therapy. J Neurosurg 75:559–563. doi:10.3171/jns.1991.75.4.0559
Sanai N, Berger MS (2011) Extent of resection influences outcomes for patients with gliomas. Rev Neurol (Paris) 167:648–654. doi:10.1016/j.neurol.2011.07.004
Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, van den Bent MJ, Chang SM (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972. doi:JCO.2009.26.354110.1200/JCO.2009.26.3541
Dhermain FG, Hau P, Lanfermann H, Jacobs AH, van den Bent MJ (2010) Advanced MRI and PET imaging for assessment of treatment response in patients with gliomas. Lancet Neurol 9:906–920. doi:S1474-4422(10)70181-210.1016/S1474-4422(10)70181-2
Galldiks N, Ullrich R, Schroeter M, Fink GR, Jacobs AH, Kracht LW (2010) Volumetry of [11C]-methionine PET uptake and MRI contrast enhancement in patients with recurrent glioblastoma multiforme. Eur J Nucl Med Mol Imaging 37:84–92. doi:10.1007/s00259-009-1219-5
Pope WB, Young JR, Ellingson BM (2011) Advances in MRI assessment of gliomas and response to anti-VEGF therapy. Curr Neurol Neurosci Rep 11:336–344. doi:10.1007/s11910-011-0179-x
Pirotte BJ, Levivier M, Goldman S, Massager N, Wikler D, Dewitte O, Bruneau M, Rorive S, David P, Brotchi J (2009) Positron emission tomography-guided volumetric resection of supratentorial high-grade gliomas: a survival analysis in 66 consecutive patients. Neurosurgery 64:471–481. doi:10.1227/01.NEU.0000338949.94496.8.00006123-200903000-00009 discussion 481
la Fougere C, Suchorska B, Bartenstein P, Kreth FW, Tonn JC (2011) Molecular imaging of gliomas with PET: opportunities and limitations. Neuro Oncol 13:806–819. doi:10.1093/neuonc/nor054
Arbizu J, Tejada S, Marti-Climent JM, Diez-Valle R, Prieto E, Quincoces G, Vigil C, Idoate MA, Zubieta JL, Penuelas I, Richter JA (2012) Quantitative volumetric analysis of gliomas with sequential MRI and [11C]-methionine PET assessment: patterns of integration in therapy planning. Eur J Nucl Med Mol Imaging 39:771–781. doi:10.1007/s00259-011-2049-9
Grosu AL, Weber WA, Riedel E, Jeremic B, Nieder C, Franz M, Gumprecht H, Jaeger R, Schwaiger M, Molls M (2005) l-methyl-[11C] methionine positron emission tomography for target delineation in resected high-grade gliomas before radiotherapy. Int J Radiat Oncol Biol Phys 63:64–74. doi:10.1016/j.ijrobp.2005.01.045
Weber DC, Zilli T, Buchegger F, Casanova N, Haller G, Rouzaud M, Nouet P, Dipasquale G, Ratib O, Zaidi H, Vees H, Miralbell R (2008) [18F]Fluoroethyltyrosine- positron emission tomography-guided radiotherapy for high-grade glioma. Radiat Oncol 3:44. doi:10.1186/1748-717X-3-44
Vees H, Senthamizhchelvan S, Miralbell R, Weber DC, Ratib O, Zaidi H (2009) Assessment of various strategies for [18F]-FET–PET-guided delineation of target volumes in high-grade glioma patients. Eur J Nucl Med Mol Imaging 36:182–193. doi:10.1007/s00259-008-0943-6
Nagahiro S, Takada A, Diksic M, Sourkes TL, Missala K, Yamamoto YL (1990) A new method to measure brain serotonin synthesis in vivo. II. A practical autoradiographic method tested in normal and lithium-treated rats. J Cereb Blood Flow Metab 10:13–21. doi:10.1038/jcbfm.1990.2
Diksic M, Nagahiro S, Sourkes TL, Yamamoto YL (1990) A new method to measure brain serotonin synthesis in vivo. I. Theory and basic data for a biological model. J Cereb Blood Flow Metab 10:1–12. doi:10.1038/jcbfm.1990.2
Muzik O, Chugani DC, Chakraborty P, Mangner T, Chugani HT (1997) Analysis of [11C]alpha-methyl-tryptophan kinetics for the estimation of serotonin synthesis rate in vivo. J Cereb Blood Flow Metab 17:659–669. doi:10.1097/00004647-199706000-00007
Chugani DC, Muzik O (2000) Alpha[11C]methyl-l-tryptophan PET maps brain serotonin synthesis and kynurenine pathway metabolism. J Cereb Blood Flow Metab 20:2–9. doi:10.1097/00004647-200001000-00002
Batista CE, Juhasz C, Muzik O, Kupsky WJ, Barger G, Chugani HT, Mittal S, Sood S, Chakraborty PK, Chugani DC (2009) Imaging correlates of differential expression of indoleamine 2,3-dioxygenase in human brain tumors. Mol Imaging Biol 11:460–466. doi:10.1007/s11307-009-0225-0
Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde BJ (2003) Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med 9:1269–1274. doi:10.1038/nm934
Munn DH, Mellor AL (2007) Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J Clin Invest 117:1147–1154. doi:10.1172/JCI31178
Miyazaki T, Moritake K, Yamada K, Hara N, Osago H, Shibata T, Akiyama Y, Tsuchiya M (2009) Indoleamine 2,3-dioxygenase as a new target for malignant glioma therapy. Laboratory investigation. J Neurosurg 111:230–237. doi:10.3171/2008.10.JNS081141
Juhasz C, Muzik O, Chugani DC, Chugani HT, Sood S, Chakraborty PK, Barger GR, Mittal S (2011) Differential kinetics of alpha-[11C]methyl-l-tryptophan on PET in low-grade brain tumors. J Neurooncol 102:409–415. doi:10.1007/s11060-010-0327-1
Juhasz C, Chugani DC, Muzik O, Wu D, Sloan AE, Barger G, Watson C, Shah AK, Sood S, Ergun EL, Mangner TJ, Chakraborty PK, Kupsky WJ, Chugani HT (2006) In vivo uptake and metabolism of alpha-[11C]methyl-l-tryptophan in human brain tumors. J Cereb Blood Flow Metab 26:345–357. doi:10.1038/sj.jcbfm.9600199
Chakraborty PK, Mangner TJ, Chugani DC, Muzik O, Chugani HT (1996) A high-yield and simplified procedure for the synthesis of alpha-[11C]methyl-l-tryptophan. Nucl Med Biol 23:1005–1008
Chugani DC, Muzik O, Chakraborty P, Mangner T, Chugani HT (1998) Human brain serotonin synthesis capacity measured in vivo with alpha-[11C]methyl-l-tryptophan. Synapse 28:33–43. doi:10.1002/(SICI)1098-2396(199801)28:1<33:AID-SYN5>3.0.CO;2-D
Kikinis R, Pieper S (2011) 3D Slicer as a tool for interactive brain tumor segmentation. Conference proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE. Eng Med Biol Soc Conf 2011:6982–6984. doi:10.1109/IEMBS.2011.6091765
Mattes D, Haynor DR, Vesselle H, Lewellen TK, Eubank W (2003) PET-CT image registration in the chest using free-form deformations. IEEE Trans Med Imaging 22:120–128. doi:10.1109/TMI.2003.809072
Bitzer M, Wockel L, Morgalla M, Keller C, Friese S, Heiss E, Meyermann R, Grote E, Voigt K (1997) Peritumoural brain oedema in intracranial meningiomas: influence of tumour size, location and histology. Acta Neurochir (Wien) 139:1136–1142. doi:10.1007/Bf01410973
Juhasz C, Chugani DC, Barger GR, Kupsky WJ, Chakraborty PK, Muzik O, Mittal S (2012) Quantitative PET imaging of tryptophan accumulation in gliomas and remote cortex: correlation with tumor proliferative activity. Clin Nucl Med 37:838–842. doi:10.1097/RLU.0b013e318251e458
Kinoshita M, Goto T, Arita H, Okita Y, Isohashi K, Kagawa N, Fujimoto Y, Kishima H, Shimosegawa E, Saitoh Y, Hatazawa J, Hashimoto N, Yoshimine T (2012) Imaging [18F]-fluorodeoxy glucose/[11C]-methionine uptake decoupling for identification of tumor cell infiltration in peritumoral brain edema. J Neurooncol 106:417–425. doi:10.1007/s11060-011-0688-0
Mittal SBA, Kupsky WJ, Kamson DO, Barger GR, Juhasz C (2012) Imaging tumor cell density in malignant gliomas: a stereotactic image-histologic analysis using tryptophan PET. Neuro Oncol 14(vi):122–123. doi:10.1093/neuonc/nos236
Tzika AA, Zarifi MK, Goumnerova L, Astrakas LG, Zurakowski D, Young-Poussaint T, Anthony DC, Scott RM, Black PM (2002) Neuroimaging in pediatric brain tumors: Gd-DTPA-enhanced, hemodynamic, and diffusion MR imaging compared with MR spectroscopic imaging. AJNR Am J Neuroradiol 23:322–333
Vaquero J, Zurita M, Morales C, Coca S (2002) Prognostic significance of tumor-enhancement and angiogenesis in oligodendroglioma. Acta Neurol Scand 106:19–23
Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280
Radbruch A, Lutz K, Wiestler B, Baumer P, Heiland S, Wick W, Bendszus M (2012) Relevance of T2 signal changes in the assessment of progression of glioblastoma according to the Response Assessment in Neurooncology criteria. Neuro Oncol 14:222–229. doi:10.1093/neuonc/nor200
Okubo S, Zhen HN, Kawai N, Nishiyama Y, Haba R, Tamiya T (2010) Correlation of l-methyl-[11C]-methionine (MET) uptake with l-type amino acid transporter 1 in human gliomas. J Neurooncol 99:217–225. doi:10.1007/s11060-010-0117-9
Fedi M, Reutens D, Okazawa H, Andermann F, Boling W, Dubeau F, White C, Nakai A, Gross DW, Andermann E, Diksic M (2001) Localizing value of alpha-methyl-l-tryptophan PET in intractable epilepsy of neocortical origin. Neurology 57:1629–1636
Juhasz C, Chugani DC, Muzik O, Shah A, Asano E, Mangner TJ, Chakraborty PK, Sood S, Chugani HT (2003) Alpha-methyl-l-tryptophan PET detects epileptogenic cortex in children with intractable epilepsy. Neurology 60:960–968
Wakamoto H, Chugani DC, Juhasz C, Muzik O, Kupsky WJ, Chugani HT (2008) Alpha-methyl-l-tryptophan positron emission tomography in epilepsy with cortical developmental malformations. Pediatr Neurol 39:181–188. doi:10.1016/j.pediatrneurol.2008.05.014
Chugani HT, Kumar A, Kupsky W, Asano E, Sood S, Juhasz C (2011) Clinical and histopathologic correlates of [11C]-alpha-methyl-l-tryptophan (AMT) PET abnormalities in children with intractable epilepsy. Epilepsia 52:1692–1698. doi:10.1111/j.1528-1167.2011.03103.x
Salber D, Stoffels G, Pauleit D, Reifenberger G, Sabel M, Shah NJ, Hamacher K, Coenen HH, Langen KJ (2006) Differential uptake of [18F]FET and [3H]l-methionine in focal cortical ischemia. Nucl Med Biol 33:1029–1035. doi:10.1016/j.nucmedbio.2006.09.004
Grosu AL, Astner ST, Riedel E, Nieder C, Wiedenmann N, Heinemann F, Schwaiger M, Molls M, Wester HJ, Weber WA (2011) An interindividual comparison of O-(2-[18F]fluoroethyl)-l-tyrosine (FET)- and l-(methyl-[11C])methionine (MET)-PET in patients with brain gliomas and metastases. Int J Radiat Oncol Biol Phys 81:1049–1058. doi:10.1016/j.ijrobp.2010.07.002
Nuutinen J, Sonninen P, Lehikoinen P, Sutinen E, Valavaara R, Eronen E, Norrgard S, Kulmala J, Teras M, Minn H (2000) Radiotherapy treatment planning and long-term follow-up with [11C]methionine PET in patients with low-grade astrocytoma. Int J Radiat Oncol Biol Phys 48:43–52
Niyazi M, Geisler J, Siefert A, Schwarz SB, Ganswindt U, Garny S, Schnell O, Suchorska B, Kreth FW, Tonn JC, Bartenstein P, la Fougere C, Belka C (2011) FET–PET for malignant glioma treatment planning. Radiother Oncol 99:44–48. doi:10.1016/j.radonc.2011.03.001
Kunz M, Thon N, Eigenbrod S, Hartmann C, Egensperger R, Herms J, Geisler J, la Fougere C, Lutz J, Linn J, Kreth S, von Deimling A, Tonn JC, Kretzschmar HA, Popperl G, Kreth FW (2011) Hot spots in dynamic [18]FET–PET delineate malignant tumor parts within suspected WHO grade II gliomas. Neuro Oncol 13:307–316. doi:10.1093/neuonc/noq196
Kinoshita M, Hashimoto N, Goto T, Yanagisawa T, Okita Y, Kagawa N, Kishima H, Tanaka H, Fujita N, Shimosegawa E, Hatazawa J, Yoshimine T (2009) Use of fractional anisotropy for determination of the cut-off value in [11C]-methionine positron emission tomography for glioma. NeuroImage 45:312–318. doi:10.1016/j.neuroimage.2008.11.034
Kracht LW, Miletic H, Busch S, Jacobs AH, Voges J, Hoevels M, Klein JC, Herholz K, Heiss WD (2004) Delineation of brain tumor extent with [11C]l-methionine positron emission tomography: local comparison with stereotactic histopathology. Clin Cancer Res 10:7163–7170. doi:10.1158/1078-0432.CCR-04-0262
Pauleit D, Floeth F, Hamacher K, Riemenschneider MJ, Reifenberger G, Muller HW, Zilles K, Coenen HH, Langen KJ (2005) O-(2-[18F]fluoroethyl)-l-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain 128:678–687. doi:10.1093/brain/awh399
Acknowledgments
The study was supported by a grant (R01 CA123451 to C.J.) from the National Cancer Institute, Start-up Funds (Wayne State University School of Medicine to S.M.) and a Strategic Research Initiative Grant from the Karmanos Cancer Institute (to S.M. and C.J.). We thank Hancheng Cai, PhD and Thomas Mangner, PhD, for assistance in PET radiochemistry. We thank Janet Barger, RN, Kelly Forcucci, RN, and Cathie Germain, MA for assisting patient recruitment and scheduling, as well as Natasha L. Robinette, MD, and Alit Yousif, MD, for reviewing the clinical MRI scans. We are grateful to the entire staff at the PET Center, Children’s Hospital of Michigan, who provided invaluable technical help in performing the PET scans.
Conflict of interest
None of the authors report any conflict of interest or financial disclosure.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kamson, D.O., Juhász, C., Buth, A. et al. Tryptophan PET in pretreatment delineation of newly-diagnosed gliomas: MRI and histopathologic correlates. J Neurooncol 112, 121–132 (2013). https://doi.org/10.1007/s11060-013-1043-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-013-1043-4