Abstract
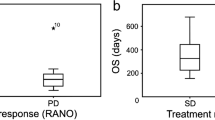
The purpose of this study was to determine whether dynamic susceptibility contrast MR perfusion relative cerebral blood volume (rCBV) correlates with prognosis of World Health Organization (WHO) grade III glial tumors and their different subtypes. Retrospective evaluation of pre-treatment tumor rCBV derived from dynamic susceptibility contrast MR perfusion was performed in 34 patients with histopathologically diagnosed WHO grade III glial tumors (anaplastic astrocytomas (n = 20), oligodendrogliomas (n = 4), and oligoastrocytomas (n = 10)). Progression free survival was correlated with rCBV using Spearman rank analysis. ROC curve analysis was performed to determine the operating point for rCBV in patients with anaplastic astrocytomas dichotomized at the median progression free survival time. For all grade III tumors (n = 34) the mean rCBV was 2.51 with a progression free survival of 705.5 days. The mean rCBV of anaplastic astrocytomas was 2.47 with progression free survival 495.2 days. In contrast, the mean rCBV for oligodendroglial tumors was 2.56 with a progression free survival of 1005.6 days. Although there was no significant correlation between rCBV and progression free survival among all types of grade III gliomas (P = 0.12), among anaplastic astrocytomas there was a significant correlation between pretreatment rCBV and progression free survival with correlation coefficient of −0.51 (P = 0.02). The operating point for rCBV in patients with anaplastic astrocytomas dichotomized at the median progression free survival time (446.5 days) was 2.86 with 78 % accuracy and there was a significant difference between the survival of patients with anaplastic astrocytomas in the dichotomized groups (P = 0.0009). Pre-treatment rCBV may serve as a prognostic imaging biomarker for anaplastic astrocytomas, but not grade III oligodendroglioma tumors.





Similar content being viewed by others
References
Kleihues P, Cavenee WK (2000) Pathology and genetics of tumours of the nervous system. IARC, Lyon
Louis DN, Ohgaki H, Wiestler OD et al (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109
Aronen HJ, Gazit IE, Louis DN et al (1994) Cerebral blood volume maps of gliomas: comparison with tumor grade and histologic findings. Radiology 191:41–51
Leon SP, Folkerth RD, Black P (1996) Microvessel density is a prognostic indicator for patients with astroglial brain tumors. Cancer 77:362–372
Von Deimling A, Louis DN, von Ammon K et al (1992) Evidence for a tumor suppressor gene on chromosome 19q associated with human astrocytomas, oligodendrogliomas, and mixed gliomas. Cancer Res 52:4277–4279
Curran WJ, Scott CB, Horton J et al (1993) Recursive partitioning analysis of prognostic factors in three radiation therapy oncology group malignant glioma trials. J Natl Cancer Inst 85:704–710
Scott SCB, Scarantino C, Urtasun R et al (1998) Validation and predictive power of radiation therapy oncology group (RTOG) recursive partitioning analysis classes for malignant glioma patients: a report using RTOG 90-06. Int J Radiat Oncol Biol Phys 40:51–55
Bruner JM, Inouye L, Fuller GN, Langford LA (1997) Diagnostic discrepancies and their clinical impact in a neuropathology referral practice. Cancer 79:796–803
Law M, Oh S, Babb JS et al (2006) Low-grade gliomas: dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging—prediction of patient clinical response. Radiology 238:658–667
Oh J, Henry RG, Pirzkall A et al (2004) Survival analysis in patients with glioblastoma multiforme: predictive value of choline-to-N-acetylaspartate index, apparent diffusion coefficient, and relative cerebral blood volume. J Magn Reson Imaging 19:546–554
Pope WB, Sayre J, Perlina A et al (2005) MR imaging correlates of survival in patients with high-grade gliomas. Am J Neuroradiol 26:2466–2474
Li Y, Lupo JM, Polley MY et al (2011) Serial analysis of imaging parameters in patients with newly diagnosed glioblastoma multiforme. Neuro Oncol 13:546–557
Haselhorst R, Kappos L, Bilecen D et al (2000) Dynamic susceptibility contrast MR imaging of plaque development in multiple sclerosis: application of an extended blood–brain barrier leakage correction. J Magn Reson Imaging 11:495–505
Wetzel SG, Cha S, Johnson G et al (2002) Relative cerebral blood volume measurements in intracranial mass lesions: interobserver and intraobserver reproducibility study. Radiology 224:797–803
Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280
Law M, Young RJ, Babb JS et al (2008) Gliomas: predicting time to progression or survival with cerebral blood volume measurements at dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology 247:490–498
Stark AM, Nabavi A, Mehdorn HM, Blomer U (2005) Glioblastoma multiforme: report of 267 cases treated at a single institution. Surg Neurol 63:162–169
Stupp R, Reni M, Gatta G, Mazza E, Vecht C (2007) Anaplastic astrocytoma in adults. Crit Rev Oncol Hematol 63:72–80
Hirai T, Murakami R, Nakamura H et al (2008) Prognostic value of perfusion MR imaging of high-grade astrocytomas: long-term follow-up study. AJNR Am J Neuroradiol 29:1505–1510
Cha S, Tihan T, Crawford F et al (2005) Differentiation of low-grade oligodendrogliomas from low-grade astrocytomas by using quantitative blood-volume measurements derived from dynamic susceptibility contrast-enhanced MR imaging. Am J Neuroradiol 26:266–273
Lev MH, Ozsunar Y, Henson JW et al (2004) Glial tumor grading and outcome prediction using dynamic spin-echo MR susceptibility mapping compared with conventional contrast-enhanced MR: confounding effect of elevated rCBV of oligodendroglimoas. AJNR Am J Neuroradiol 25:214–221
Bisdas S, Kirkpatrick M, Giglio P et al (2009) Cerebral blood volume measurements by perfusion-weighted MR imaging in gliomas: ready for prime time in predicting short-term outcome and recurrent disease? AJNR Am J Neuroradiol 30:681–688
Schiffer D, Dutto A, Cavalla P et al (1997) Prognostic factors in oligodendroglioma. Can J Neurol Sci 24:313–319
Mork SJ, Halvorsen TB, Lindegaard KF, Eide GE (1986) Oligodendroglioma: histologic evaluation and prognosis. J Neuropathol Exp Neurol 45:65–78
Engelhard HH, Stelea A, Mundt A (2003) Oligodendroglioma and anaplastic oligodendroglioma: clinical features, treatment, and prognosis. Surg Neurol 60:443–456
Whitmore RG et al (2007) Prediction of oligodendroglial tumor subtype and grade using perfusion weighted magnetic resonance imaging. J Neurosurg 107:600–609
Christov C, Adle-Biassette H, Le Guerinel C et al (1998) Immunohistochemical detection of vascular endothelial growth factor (VEGF) in the vasculature of oligodendrogliomas. Neuropathol Appl Neurobiol 24:29–35
Lafuente JV, Adán B, Alkiza K, Garibi JM, Rossi M, Cruz-Sánchez FF (1999) Expression of vascular endothelial growth factor (VEGF) and platelet-derived growth factor receptor-beta (PDGFR-beta) in human gliomas. J Mol Neurosci 13:177–185
Pietsch T, Valter MM, Wolf HK et al (1997) Expression and distribution of vascular endothelial growth factor protein in human brain tumors. Acta Neuropathol 93:109–117
Maia AC Jr, Malheiros SM, da Rocha AJ et al (2005) MR cerebral blood volume maps correlated with vascular endothelial growth factor expression and tumor grade in nonenhancing gliomas. AJNR Am J Neuroradiol 26:777–783
Netto GC, Bleil CB, Hilbig A et al (2008) Immunohistochemical evaluation of the microvascular density through the expression of TGF-b (CD 105/endoglin) and CD 34 receptors and expression of the vascular endothelial growth factor (VEGF) in oligodendrogliomas. Neuropathology 28:17–23
Boxerman J, Schmainda KM, Weisskoff RM (2006) Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade whereas uncorrected maps do not. AJNR Am J Neuroradiol 27:859–867
Conflict of interest
None of the authors have conflicts of interests or any relevant disclosures.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mangla, R., Ginat, D.T., Kamalian, S. et al. Correlation between progression free survival and dynamic susceptibility contrast MRI perfusion in WHO grade III glioma subtypes. J Neurooncol 116, 325–331 (2014). https://doi.org/10.1007/s11060-013-1298-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-013-1298-9