Abstract
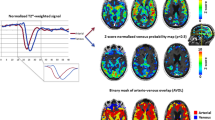
Abnormal brain tumor vasculature has recently been highlighted by a dynamic susceptibility contrast (DSC) MRI processing technique. The technique uses independent component analysis (ICA) to separate arterial and venous perfusion. The overlap of the two, i.e. arterio-venous overlap or AVOL, preferentially occurs in brain tumors and predicts response to anti-angiogenic therapy. The effects of contrast agent leakage on the AVOL biomarker have yet to be established. DSC was acquired during two separate contrast boluses in ten patients undergoing clinical imaging for brain tumor diagnosis. Three components were modeled with ICA, which included the arterial and venous components. The percentage of each component as well as a third component were determined within contrast enhancing tumor and compared. AVOL within enhancing tumor was also compared between doses. The percentage of enhancing tumor classified as not arterial or venous and instead into a third component with contrast agent leakage apparent in the time-series was significantly greater for the first contrast dose compared to the second. The amount of AVOL detected within enhancing tumor was also significantly greater with the second dose compared to the first. Contrast leakage results in large signal variance classified as a separate component by the ICA algorithm. The use of a second dose mitigates the effect and allows measurement of AVOL within enhancement.




Similar content being viewed by others
References
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109
Deane BR, Lantos PL (1981) The vasculature of experimental brain tumours. Part 1. A sequential light and electron microscope study of angiogenesis. J Neurol Sci 49:55–66
Folkman J (1990) What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst 82:4–6
Millauer B, Shawver LK, Plate KH, Risau W, Ullrich A (1994) Glioblastoma growth inhibited in vivo by a dominant-negative Flk-1 mutant. Nature 367:576–579
Leon SP, Folkerth RD, Black PM (1996) Microvessel density is a prognostic indicator for patients with astroglial brain tumors. Cancer 77:362–372
Lev MH, Rosen BR (1999) Clinical applications of intracranial perfusion MR imaging. Neuroimaging Clin N Am 9:309–331
Cha S, Lu S, Johnson G, Knopp EA (2000) Dynamic susceptibility contrast MR imaging: correlation of signal intensity changes with cerebral blood volume measurements. J Magn Reson Imaging 11:114–119
Covarrubias DJ, Rosen BR, Lev MH (2004) Dynamic magnetic resonance perfusion imaging of brain tumors. Oncologist 9:528–537
Gahramanov S, Raslan AM, Muldoon LL, Hamilton BE, Rooney WD, Varallyay CG, Njus JM, Haluska M, Neuwelt EA (2011) Potential for differentiation of pseudoprogression from true tumor progression with dynamic susceptibility-weighted contrast-enhanced magnetic resonance imaging using ferumoxytol vs. gadoteridol: a pilot study. Int J Radiat Oncol Biol Phys 79:514–523
Barajas RF Jr, Chang JS, Segal MR, Parsa AT, McDermott MW, Berger MS, Cha S (2009) Differentiation of recurrent glioblastoma multiforme from radiation necrosis after external beam radiation therapy with dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology 253:486–496
Hoefnagels FW, Lagerwaard FJ, Sanchez E, Haasbeek CJ, Knol DL, Slotman BJ, Vandertop WP (2009) Radiological progression of cerebral metastases after radiosurgery: assessment of perfusion MRI for differentiating between necrosis and recurrence. J Neurol 256:878–887
Hu LS, Baxter LC, Smith KA, Feuerstein BG, Karis JP, Eschbacher JM, Coons SW, Nakaji P, Yeh RF, Debbins J, Heiserman JE (2009) Relative cerebral blood volume values to differentiate high-grade glioma recurrence from posttreatment radiation effect: direct correlation between image-guided tissue histopathology and localized dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging measurements. AJNR Am J Neuroradiol 30:552–558
Sugahara T, Korogi Y, Tomiguchi S, Shigematsu Y, Ikushima I, Kira T, Liang L, Ushio Y, Takahashi M (2000) Posttherapeutic intraaxial brain tumor: the value of perfusion-sensitive contrast-enhanced MR imaging for differentiating tumor recurrence from nonneoplastic contrast-enhancing tissue. AJNR Am J Neuroradiol 21:901–909
LaViolette PS, Cohen AD, Prah MA, Rand SD, Connelly J, Malkin MG, Mueller WM, Schmainda KM (2013) Vascular change measured with independent component analysis of dynamic susceptibility contrast MRI predicts bevacizumab response in high-grade glioma. Neuro Oncol 15:442–450
Paulson ES, Schmainda KM (2008) Comparison of dynamic susceptibility-weighted contrast-enhanced MR methods: recommendations for measuring relative cerebral blood volume in brain tumors. Radiology 249:601–613
Beckmann CF, Smith SM (2004) Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging 23:137–152
Hyvarinen A (1999) Fast and robust fixed-point algorithms for independent component analysis. IEEE Trans Neural Netw 10:626–634
LaViolette PS, Cohen AD, Schmainda KM (2012) Contrast leakage in high grade glioma measured with independent component analysis of dynamic susceptibility contrast MRI. Proc ISMRM, Melbourne
Cox RW (1996) AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29:162–173
Law M, Yang S, Wang H, Babb JS, Johnson G, Cha S, Knopp EA, Zagzag D (2003) Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am J Neuroradiol 24:1989–1998
Knopp EA, Cha S, Johnson G, Mazumdar A, Golfinos JG, Zagzag D, Miller DC, Kelly PJ, Kricheff II (1999) Glial neoplasms: dynamic contrast-enhanced T2*-weighted MR imaging. Radiology 211:791–798
Aronen HJ, Gazit IE, Louis DN, Buchbinder BR, Pardo FS, Weisskoff RM, Harsh GR, Cosgrove GR, Halpern EF, Hochberg FH et al (1994) Cerebral blood volume maps of gliomas: comparison with tumor grade and histologic findings. Radiology 191:41–51
Sugahara T, Korogi Y, Shigematsu Y, Liang L, Yoshizumi K, Kitajima M, Takahashi M (1999) Value of dynamic susceptibility contrast magnetic resonance imaging in the evaluation of intracranial tumors. Top Magn Reson Imaging 10:114–124
Sugahara T, Korogi Y, Kochi M, Ikushima I, Hirai T, Okuda T, Shigematsu Y, Liang L, Ge Y, Ushio Y, Takahashi M (1998) Correlation of MR imaging-determined cerebral blood volume maps with histologic and angiographic determination of vascularity of gliomas. AJR Am J Roentgenol 171:1479–1486
Wong ET, Jackson EF, Hess KR, Schomer DF, Hazle JD, Kyritsis AP, Jaeckle KA, Yung WK, Levin VA, Leeds NE (1998) Correlation between dynamic MRI and outcome in patients with malignant gliomas. Neurology 50:777–781
Boxerman JL, Schmainda KM, Weisskoff RM (2006) Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. AJNR Am J Neuroradiol 27:859–867
Hu LS, Baxter LC, Pinnaduwage DS, Paine TL, Karis JP, Feuerstein BG, Schmainda KM, Dueck AC, Debbins J, Smith KA, Nakaji P, Eschbacher JM, Coons SW, Heiserman JE (2010) Optimized preload leakage-correction methods to improve the diagnostic accuracy of dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging in posttreatment gliomas. AJNR Am J Neuroradiol 31:40–48
Spampinato MV, Wooten C, Dorlon M, Besenski N, Rumboldt Z (2006) Comparison of first-pass and second-bolus dynamic susceptibility perfusion MRI in brain tumors. Neuroradiology 48:867–874
McKeown MJ, Makeig S, Brown GG, Jung TP, Kindermann SS, Bell AJ, Sejnowski TJ (1998) Analysis of fMRI data by blind separation into independent spatial components. Hum Brain Mapp 6:160–188
Calhoun VD, Liu J, Adali T (2009) A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. Neuroimage 45:S163–S172
Onton J, Westerfield M, Townsend J, Makeig S (2006) Imaging human EEG dynamics using independent component analysis. Neurosci Biobehav Rev 30:808–822
Kao YH, Guo WY, Wu YT, Liu KC, Chai WY, Lin CY, Hwang YS (2003) Jy-Kang Liou A, Wu HM, Cheng HC, Yeh TC, Hsieh JC, Mu Huo Teng M: Hemodynamic segmentation of MR brain perfusion images using independent component analysis, thresholding, and Bayesian estimation. Magn Reson Med 49:885–894
LaViolette PS, Cohen AD, Rand SD, Mueller WM, Schmainda KM (2011) Independent component analysis of dynamic susceptibility contrast MRI in brain tumor: a new biomarker for measuring tumor perfusion patterns. Proc ISMRM, Montreal Quebec
Senger DR, Van de Water L, Brown LF, Nagy JA, Yeo KT, Yeo TK, Berse B, Jackman RW, Dvorak AM, Dvorak HF (1993) Vascular permeability factor (VPF, VEGF) in tumor biology. Cancer Metastasis Rev 12:303–324
LaViolette PS, Daun M, Cohen AD, Connelly J, Schmainda KM (2013) Optimizing repeatability of independent component analysis applied to dynamic susceptibility contrast MRI in 68 brain tumor patients with five repeated scans. In: Proceedings of the 21st Annual Meeting of ISMRM Salt Lake City
LaViolette PS, Daun M, Prah M, Jafari-Khouzani K, Polaskova P, Gerstner ER, Stufflebeam SM, Schmainda KM (2013) Repeatability of Independent Component Analysis applied to Dynamic Susceptibility Contrast MRI in Newly Diagnosed Brain Tumor Patients with Two Baseline Imaging Scans. In: Proceedings of the 21st Annual Meeting of ISMRM Salt Lake City
Acknowledgments
Special thanks to the patients who chose to participate in this study. This work was funded by NIH/NCI R01CA082500, and Advancing a Healthier Wisconsin.
Conflict of interest
All authors report no conflict of interest.
Ethical standards
This research was performed in approval of our institutional review board.
Author information
Authors and Affiliations
Corresponding author
Additional information
Peter S. LaViolette and Mitchell K. Daun contributed equally to this work.
Rights and permissions
About this article
Cite this article
LaViolette, P.S., Daun, M.K., Paulson, E.S. et al. Effect of contrast leakage on the detection of abnormal brain tumor vasculature in high-grade glioma. J Neurooncol 116, 543–549 (2014). https://doi.org/10.1007/s11060-013-1318-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-013-1318-9